Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: ANCA-associated vasculitis (AAV) imposes a high morbidity burden, both from the disease itself and from treatment-related side effects, often compromising patients’ quality of life (QoL). Despite this, patient-reported outcomes (PRO) remain insufficiently studied compared to physician-reported measures. We present initial PRO data from a prospective, longitudinal cohort of individuals with AAV.
Methods: The Mass General Brigham AAV Prospective Registry (AAVPR) enrolls patients with GPA, MPA and EGPA at any stage of disease and regardless of disease activity status. Participants complete surveys every 6 months capturing treatments, comorbidities, and PROs, including the SF-12 and the AAV-PRO, among other variables. The SF-12 gathers physical and mental healthdata in 8 domains and provides physical and mental component summaries (PCS, MCS; 0-100 with lower scores indicating worse QoL). The AAV-PRO collects information in 6 domains (0-100 with higher scores indicating worse QoL and disease burden), including organ-specific symptoms (OSS), systemic-symptoms (SS), treatment side-effects (TSE), social and emotional impact (SEI), concerns about the future (CAF) and physical function (PF). We performed multivariable-adjusted regression to identify factors associated with PROs at baseline and multivariable-adjusted difference-in-difference (DID) regression to identify factors associated with change in PROs from baseline to 6 months. Covariates considered included age, sex, disease duration, glucocorticoid (GC) and immunosuppression use within 6 months prior to baseline, ANCA type and multimorbidity.
Results: Among 92 participants with ≥6 months of follow-up, the mean (SD) age was 59.9 (14.5) years, and the median (IQR) duration of disease at baseline was 38 (4-79.5) months. The cohort was predominantly female (69.6%) and White (94.6%), with 38% MPO-ANCA positive patients. Most common AAV treatments within 6 months prior to baseline included GCs (43.5%) and rituximab (54.4%). Additional baseline features are in Table 1. Over 6 months, the SF-12 PCS and MCS, and most AAV-PRO domains remained relatively stable, except for SEI scores, which significantly improved (Table 2). At baseline (Table 3), females reported worse AAV-PRO OSS and TSE scores compared to males. Patients with disease duration ≥6 months had better baseline AAV-PRO OSS, TSE, and PF scores than those with shorter disease duration. Over a 6-month period (Table 3), PR3-positive patients showed improvement in AAV-PRO SS, whereas MPO-positive patients worsened (baseline to 6 months mean difference -5.8 for PR3 vs 5.9 for MPO). Additionally, patients with disease duration ≥6 months experienced less improvement in AAV-PRO SEI (baseline to 6 months mean difference -2.8 for ≥6 months vs -11.7 for < 6 months) and a decline in SF-12 MCS (baseline to 6 months mean difference -0.3 for ≥6 months vs 5.3 for < 6 months) compared to those with shorter disease duration. No other statistically significant associations were found.
Conclusion: In this prospective cohort of patients with AAV, sex, disease duration, and ANCA type demonstrated independent effects on PRO including the AAV-PRO and the SF-12
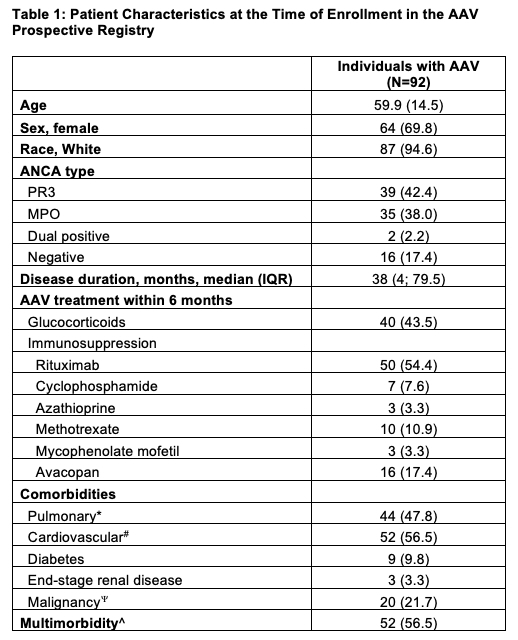 Values are n (%) for categorical variables and mean (SD) for continuous variables unless otherwise specified. *Including chronic obstructive pulmonary disease, sleep apnea, bronchiectasis, interstitial lung disease and/or asthma. #Including hypertension, myocardial infarction, coronary artery disease undergoing revascularization, carotid artery stenosis, peripheral artery disease, atrial fibrillation, heart failure and/or stroke. Including gastrointestinal, lung, breast, urogenital, hematologic, bone and soft tissue, brain, skin, and/or thyroid malignancy. ^≥1 Comorbidity. AAV, ANCA-associated vasculitis.
Values are n (%) for categorical variables and mean (SD) for continuous variables unless otherwise specified. *Including chronic obstructive pulmonary disease, sleep apnea, bronchiectasis, interstitial lung disease and/or asthma. #Including hypertension, myocardial infarction, coronary artery disease undergoing revascularization, carotid artery stenosis, peripheral artery disease, atrial fibrillation, heart failure and/or stroke. Including gastrointestinal, lung, breast, urogenital, hematologic, bone and soft tissue, brain, skin, and/or thyroid malignancy. ^≥1 Comorbidity. AAV, ANCA-associated vasculitis.
.jpg) Values are mean (SD). The AAV-PRO ranges 0-100 with higher scores indicating worse quality of life and disease burden. The SF-12 ranges 0-100 with lower scores indicating worse quality of life. AAV, ANCA-associated vasculitis; PRO, patient-reported outcome
Values are mean (SD). The AAV-PRO ranges 0-100 with higher scores indicating worse quality of life and disease burden. The SF-12 ranges 0-100 with lower scores indicating worse quality of life. AAV, ANCA-associated vasculitis; PRO, patient-reported outcome
.jpg) ▴Within 6 months prior to baseline. β, linear regression beta coefficient. Bolded estimates indicate P values < 0.05. AAV, ANCA-associated vasculitis; PRO, patient-reported outcome; OLS, ordinary least squares; SF-12, 12-Item Short Form Inventory Survey; MCS, mental component summary. AAV-PRO domains: OSS, organ-specific symptoms; SS, systemic symptoms; TSE, treatment side-effects; SEI, social and emotional impact; PF, physical function.
▴Within 6 months prior to baseline. β, linear regression beta coefficient. Bolded estimates indicate P values < 0.05. AAV, ANCA-associated vasculitis; PRO, patient-reported outcome; OLS, ordinary least squares; SF-12, 12-Item Short Form Inventory Survey; MCS, mental component summary. AAV-PRO domains: OSS, organ-specific symptoms; SS, systemic symptoms; TSE, treatment side-effects; SEI, social and emotional impact; PF, physical function.
To cite this abstract in AMA style:
Johnson C, Shepherd N, O'Dea D, King A, Katz G, Arevalo Molina B, Williams Z, Negron M, Patel N, Unizony S. Patient-Reported Outcomes in ANCA-Associated Vasculitis: Early Findings from a Prospective Real-World Cohort [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/patient-reported-outcomes-in-anca-associated-vasculitis-early-findings-from-a-prospective-real-world-cohort/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patient-reported-outcomes-in-anca-associated-vasculitis-early-findings-from-a-prospective-real-world-cohort/
