Session Information
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: COMPACT is a non-interventional study evaluating the persistence, effectiveness, safety and patient-reported outcomes (PROs) in patients with rheumatoid arthritis (RA), axial-spondyloarthritis or psoriatic arthritis treated with GP2015 (an etanercept [ETN] biosimilar) in real-world conditions.1 Here we present the PROs and quality of life (QoL) in RA patients treated with GP2015, final results from the COMPACT study.
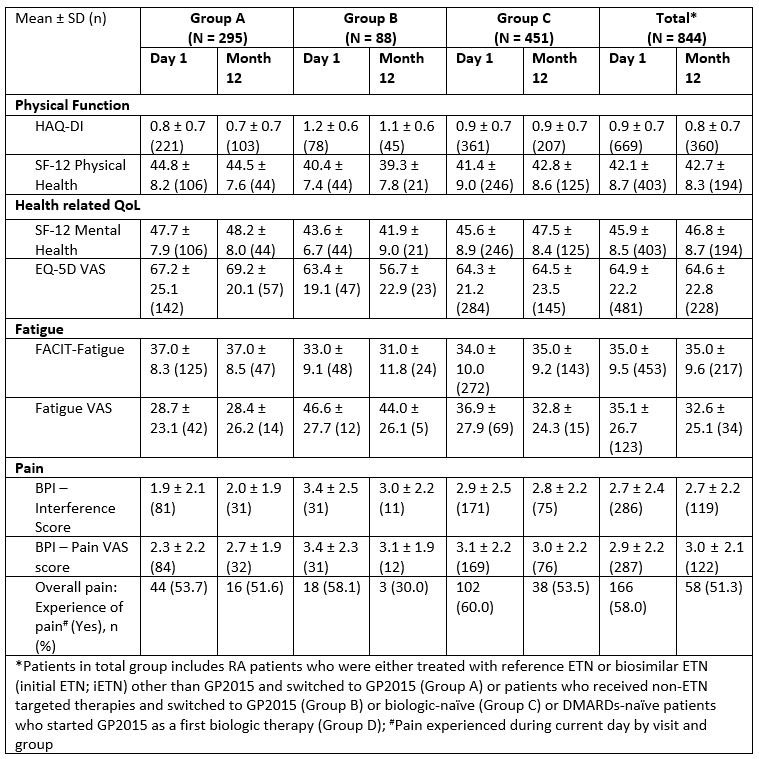
Methods: Patients with RA aged ≥18 years who initiated treatment with GP2015 were enrolled in this study.1 Patients were categorized based on prior treatment status: patients with clinical remission or low disease activity under treatment with reference ETN or biosimilar ETN (iETN) and switched to GP2015 (Group A) or patients who received non-ETN targeted therapies and switched to GP2015 (Group B) or biologic-naïve patients who started GP2015 after conventional therapy failure (Group C) or disease modifying anti-rheumatic drug (DMARD)-naïve patients with recent diagnosis of RA considered suitable for treatment initiation with a biologic and started on treatment with GP2015 (Group D). PROs and QoL were assessed using health assessment questionnaire-disability index (HAQ-DI), short form health survey 12-item (SF-12)-physical/mental health, EuroQol-5D overall self-rated health status (EQ-5D visual analogue scale [VAS]), functional assessment of chronic illness therapy (FACIT)-fatigue, fatigue VAS, basic pain inventory (BPI)-interference score, pain VAS scores and overall pain (experience of pain during current day) scores.
Results: The recruited patients had an ongoing GP2015 treatment at the time of enrolment for an average of 138 days. A total of 844 RA patients were observed, of whom 73.0% (n = 616) were female. The mean age and BMI of patients enrolled in the study were 57.9 years and 27.1kg/m2, respectively. The total number of patients enrolled to Groups A, B, C and D were 295, 88, 451 and 10, respectively. PRO and QoL for patients in Groups A, B and C were assessed in terms of physical function, health related QoL, fatigue and pain. The results showed comparable scores between Group A (switched patients) and Group C (biologic-naïve patients) through 12 months of treatment with GP2015 in RA patients. Numerically different scores were observed in Group B (patients who received non-ETN targeted therapies and switched to GP2015) (Table). No results were analyzed for patients in Group D due to low number of patients (n = 10). Across all patient groups, no major differences were observed between baseline and Month 12, which may be explained by ongoing GP2015 treatment at the time of enrolment.
Conclusion: This final analysis showed comparable PRO and QoL scores obtained after 12 months of observation in patients with RA who switched from iETN to GP2015 and patients who were biologic-naïve and started GP2015 after conventional therapy failure.
Reference
- Schmalzing M, et al. ACR 2019, Atlanta, GA, United States. Poster No. 553.
To cite this abstract in AMA style:
Askari A, Schmalzing M, Kellner H, Ortega-Castro R, Perez-Coleman J, Rosario F, Jeka S, Haraoui B, Allanore Y, Rahman M, Furlan F, Hachaichi S, Sheeran T. Patient-reported Outcomes and Quality of Life in Patients with Rheumatoid Arthritis Treated with GP2015: Final Results from a Real-world Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/patient-reported-outcomes-and-quality-of-life-in-patients-with-rheumatoid-arthritis-treated-with-gp2015-final-results-from-a-real-world-study/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patient-reported-outcomes-and-quality-of-life-in-patients-with-rheumatoid-arthritis-treated-with-gp2015-final-results-from-a-real-world-study/