Session Information
Date: Sunday, November 17, 2024
Title: SLE – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Voclosporin used in addition to Mycophenolate Mofetil and low dose oral steroids in patients with active Lupus Nephritis (LN) was found to be superior to Placebo in Phase 3, global, double blind, randomized control trial (AURORA 1). Herein we report the post-hoc analysis results of patient reported outcome (PRO) measures used in this clinical trial. LIT is short 10 item unidimensional, while LupusPRO is multidimensional with 43 items.
Methods: 357 patients with biopsy proven active LN were randomized to receive voclosporin 23.7 mg BID or a placebo, in addition to background standard of care. Primary Outcome of interest was complete renal response (CRR) at week 52. PRO measurements were obtained at baseline, 12-, 24- and 52-weeks using Lupus Impact Tracker (LIT), LupusPRO V1.7 and SF36. We compared magnitude and direction of changes in LIT, LupusPRO domains and SF-36, between (a) those that achieved CRR (responders) and that did not (non-responders) at week 52 as compared to baseline, and (b) those receiving standard of Care vs Voclosporin weeks 12, 24 and 52 as compared to baseline. T tests were used to make comparisons with a p value ≤0.05 considered significant.
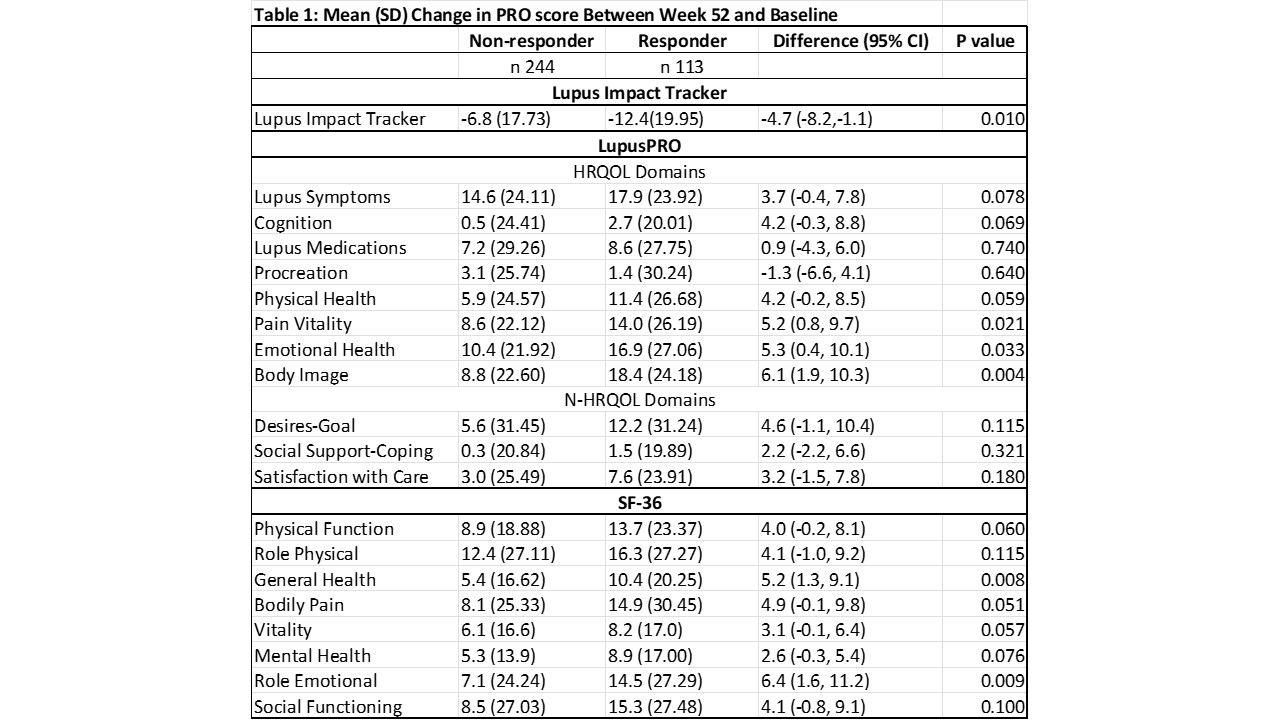
Results: Forty (40.8%) of patients on Voclosporin and 22.5% on Placebo achieved CRR at week 52 (OR 2.65, 95% CI 1.64,4.27, P< 0.001). A significant reduction in LIT score was seen among responders and non-responders (Mean reduction -11.8 vs -7.1). The mean difference in LIT scores among the two groups was -4.7 (95% CI -8.2, -1.1, p 0.010), and exceeded the minimally important difference for LIT of 4. Significant improvements in LupusPRO domains Pain-Vitality (mean difference 5.2, p 0.02), Emotional Health (mean difference 5.3, p 0.03) and Body Image (6.1, p 0.0048) were observed among responders than responders at week 52, while trends towards improvements were noted for Lupus symptoms, Cognition and Physical Health LupusPRO domains (Table 1). Similarly significant improvements were noted on SF-36 domains of Role Emotional (mean difference 6.4, p 0.009) and General Health (mean difference 5.2, p 0.008).
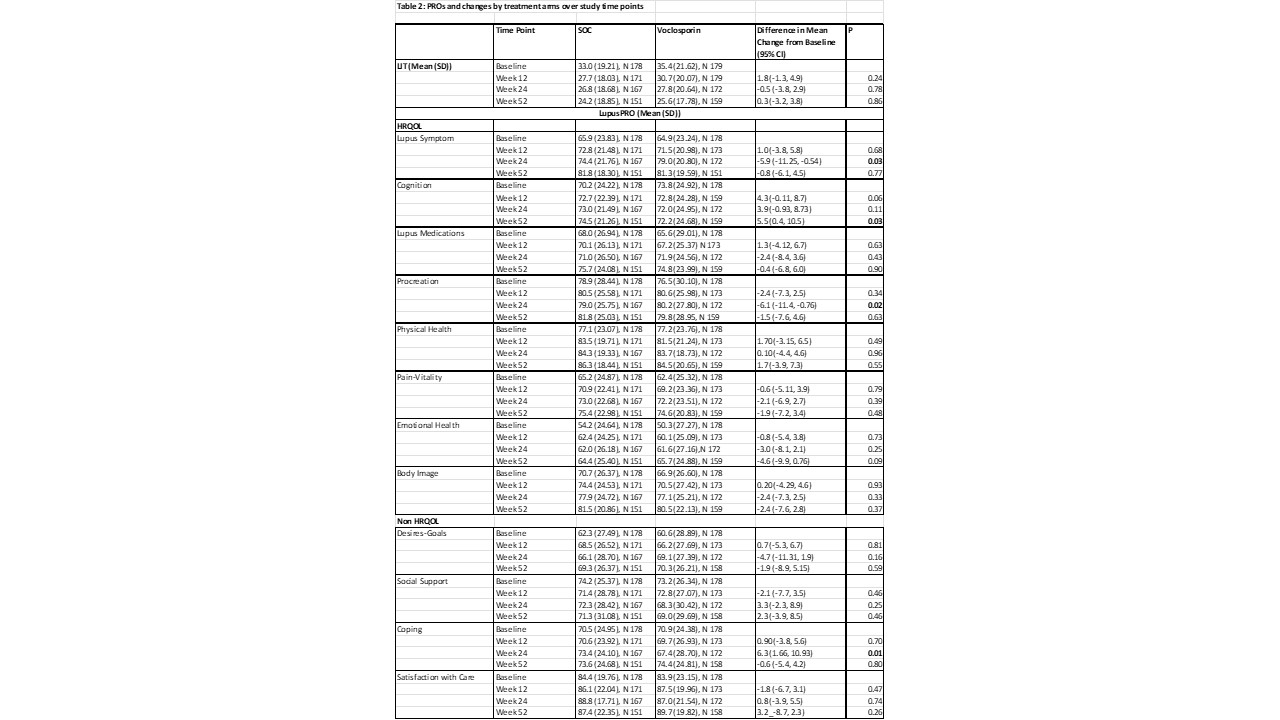
No significant differences in LIT or LupusPRO domains (except Lupus Symptom, Lupus Medication, Procreation domains) were noted over time in the two groups when comparing those receiving SOC vs Voclosporin (Table 2).
Conclusion: Significant improvements in PROs (LIT and LupusPRO HRQOL domains) were noted among responders and non-responders in the AURORA 1 trial. Significantly larger magnitude and clinically meaningful reductions in negative impacts of lupus on patients’ daily lives were noted on the Lupus Impact Tracker among patients that attained CRR as compared to those that did not. LIT or LupusPRO HRQOL domains capture more proximal impacts of the disease or its treatments in clinical trials. LupusPRO non HRQOL domains may be more suited to routine patient care to evaluate distal impacts of the disease, internal-external resources available to the patient, and their satisfaction with care.
To cite this abstract in AMA style:
Jolly M, Truman M, Flauto R, Dao K. Patient Reported Outcomes Analyses from AURORA 1 Clinical Trial: Lupus Impact Tracker and LupusPRO [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/patient-reported-outcomes-analyses-from-aurora-1-clinical-trial-lupus-impact-tracker-and-lupuspro/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patient-reported-outcomes-analyses-from-aurora-1-clinical-trial-lupus-impact-tracker-and-lupuspro/