Session Information
Date: Monday, November 8, 2021
Title: Patient Outcomes, Preferences, & Attitudes Poster III: Patient Preferences (1153–1169)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: The use of biosimilars instead of its originator is a controversial subject with many implications. It is considered that a non medical switch should not occur and that pts and physicians must be involved in the decision of choosing the adequate medication.
Methods: A social media survey via The Mexican Foundation for Rheumatic Patients (FUMERAC) (Facebook [FB] and Twitter) was conducted in Mexico from November 2020 to January 2021. An opening question to ascertain the consent Pts were eligible if they were >18 years of age and if they had any inflammatory or non inflammatory rheumatic condition and the use of biologics/biosimilars was not mandatory.
Results: A total of 165 pts completed the survey. 81% were women and 79% had high school or higher education. The most frequent diagnoses were RA (56.4%), AS (13.9%), PsA (11.5%) and Lupus (9.1%). DMARD monotherapy was the most common treatment representing 30% of pts. 57% reported prior or current use of biologics. Sixty four percent of pts had never heard the term biosimilar. 38% would accept the change from an originator to its biosimilar if the opinion of the patient and physician was taken into account. 24% of pts would take a legal measure or file a complaint if a NMS was to happen. The subjects asked to pts in order to reduce their inconformity were: treatment effectiveness (65.5%), adverse effects (60%), reason for change (54.4%), treatment duration (29%) and other pts experience (19.3%)
Conclusion: In Mexico the concept of biosimilars is barely known. Most of patients (76%) would not take any measure if they where changed from an originator to its biosimilar. There is still a great amount of confidence from the pts to their physician regarding the management of their medications.
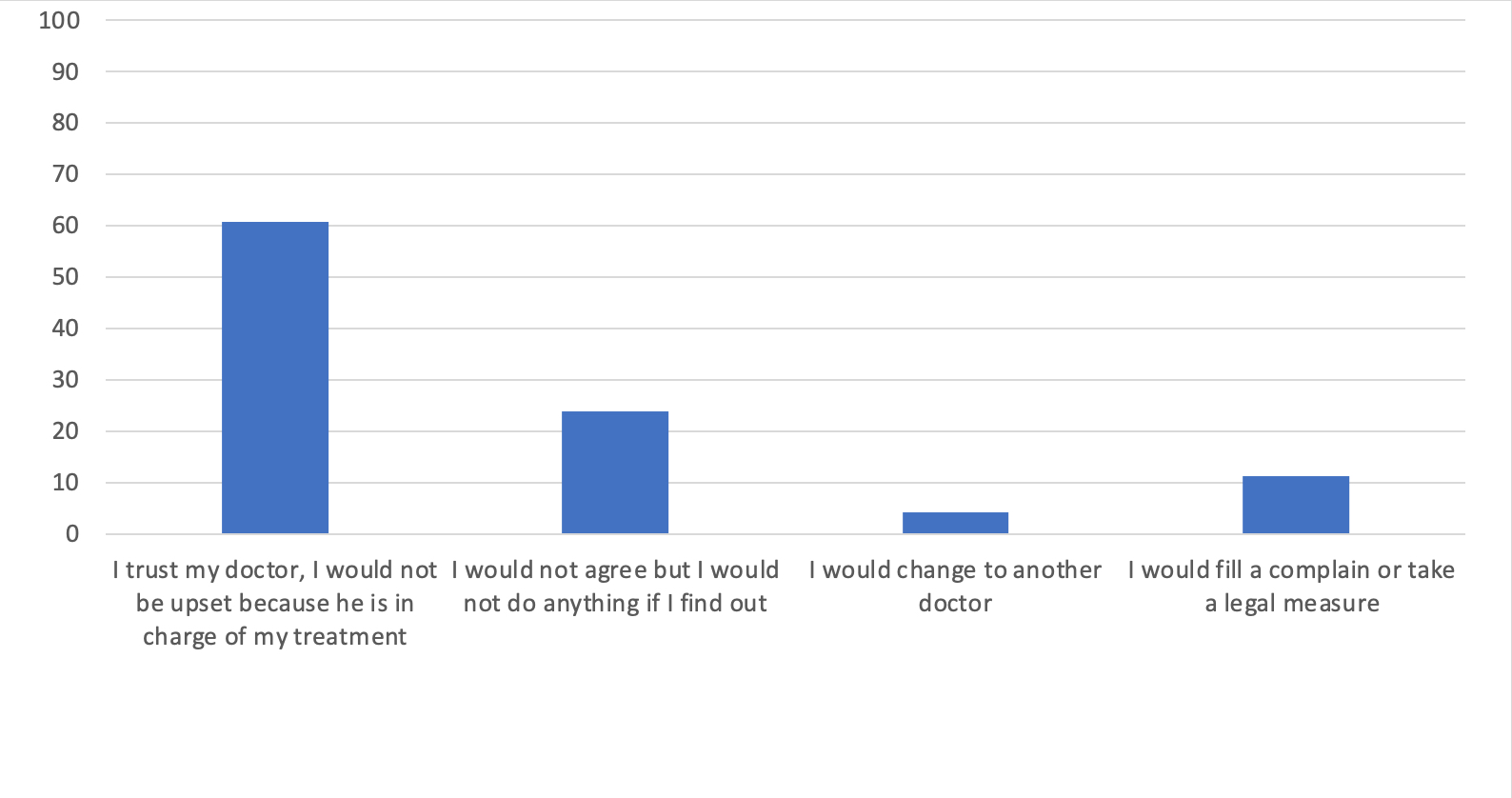 What would you think if your physician changed your originator to its biosimilar without asking nor explaining you?
What would you think if your physician changed your originator to its biosimilar without asking nor explaining you?
 What would you think if your pharmacist or health insurance policy changed your medication to its biosimilar without informing you or your physician?
What would you think if your pharmacist or health insurance policy changed your medication to its biosimilar without informing you or your physician?
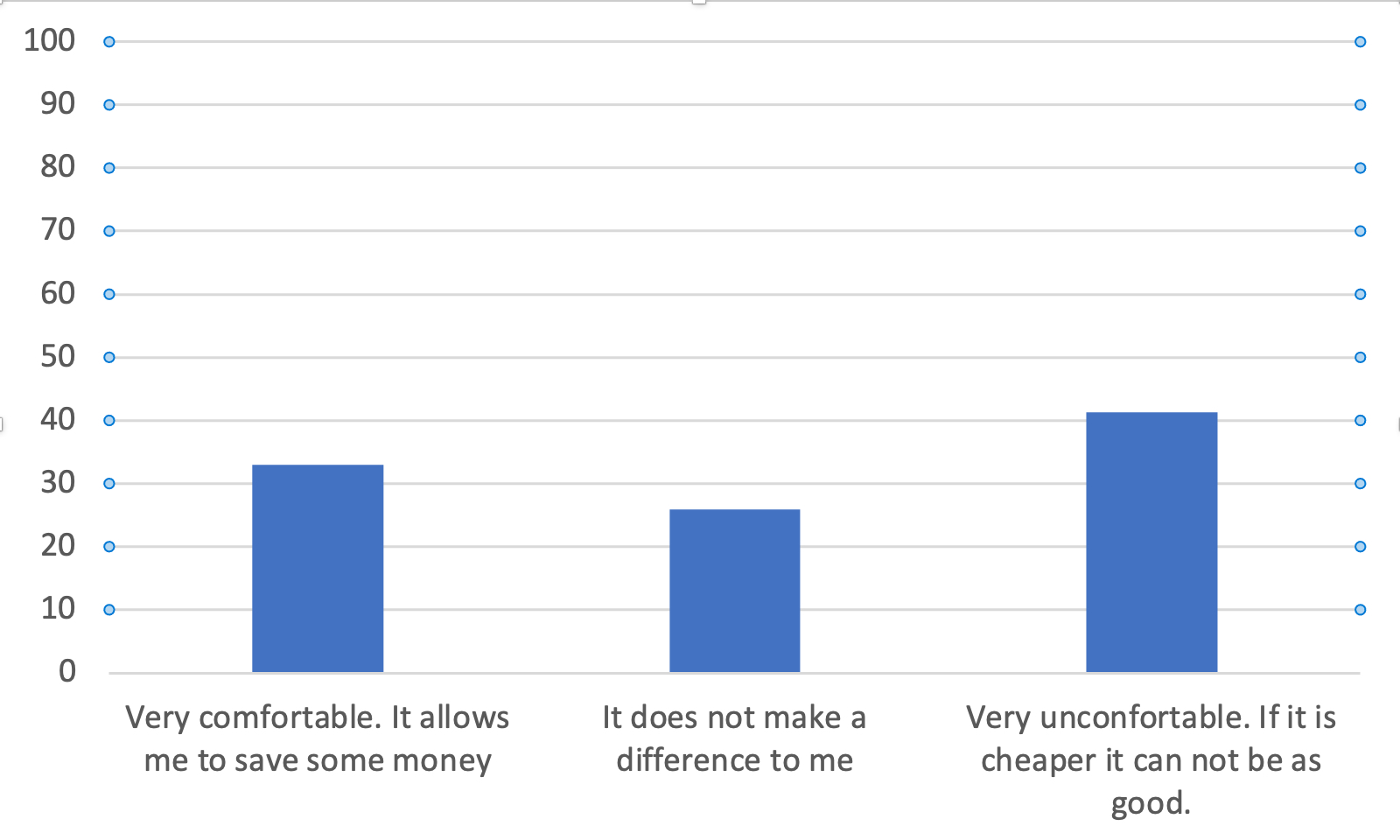 Do you think changing your biologic drug to its more economic biosimilar can have a consequence?
Do you think changing your biologic drug to its more economic biosimilar can have a consequence?
To cite this abstract in AMA style:
Vega Morales D, Garza-Alpirez A, Díaz-Garza C. Patient Perspective on the Non Medical Switch of Originator to Its Biosimilar in Inflammatory Arthritis Using a Social Media Survey [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/patient-perspective-on-the-non-medical-switch-of-originator-to-its-biosimilar-in-inflammatory-arthritis-using-a-social-media-survey/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patient-perspective-on-the-non-medical-switch-of-originator-to-its-biosimilar-in-inflammatory-arthritis-using-a-social-media-survey/
