Session Information
Date: Monday, November 9, 2020
Session Type: Abstract Session
Session Time: 11:00AM-11:50AM
Background/Purpose: Characterization of how different types of patient-generated data reflect patients’ experience is needed to guide integration of electronically collected patient-reported outcome (ePRO) measures and passive biometrics into real-word evidence (RWE) platforms. Our objective was to characterize engagement, protocol adherence, and data completeness in an ongoing study in rheumatoid arthritis (RA) participants (pts) enrolled in the Digital Tracking of Arthritis Longitudinally (DIGITAL) study, an ancillary study of the ArthritisPower® registry.
Methods: Pts were invited to join the app-based study which included a 2-week (14-day) Lead-In and 12-week (84-day) Main Study Period. Study-specific customization of the ArthritisPower mobile application collected ePROs. For at least 10 days of the Lead-In period, pts were required to electronically complete: a) two daily single-item Pain and Fatigue numeric rating scales and b) longer weekly sets of ePROs. Successful completers of the Lead-In were mailed a smartwatch (Fitbit® Versa™) and study materials. Main Study Period included automated and manual prompts to complete ePROs, wear the smartwatch and regularly sync it. Study coordinators monitored pt data and contacted pts via email, text and/or phone to resolve adherence issues per a priori rules triggering pt contact due to consecutive spans of missing data. Adherence to data collection during the Main Study was defined as providing requested data > 70% of 84 days (daily ePRO, smartwatch data), or ≥ 9 of 12 weeks.
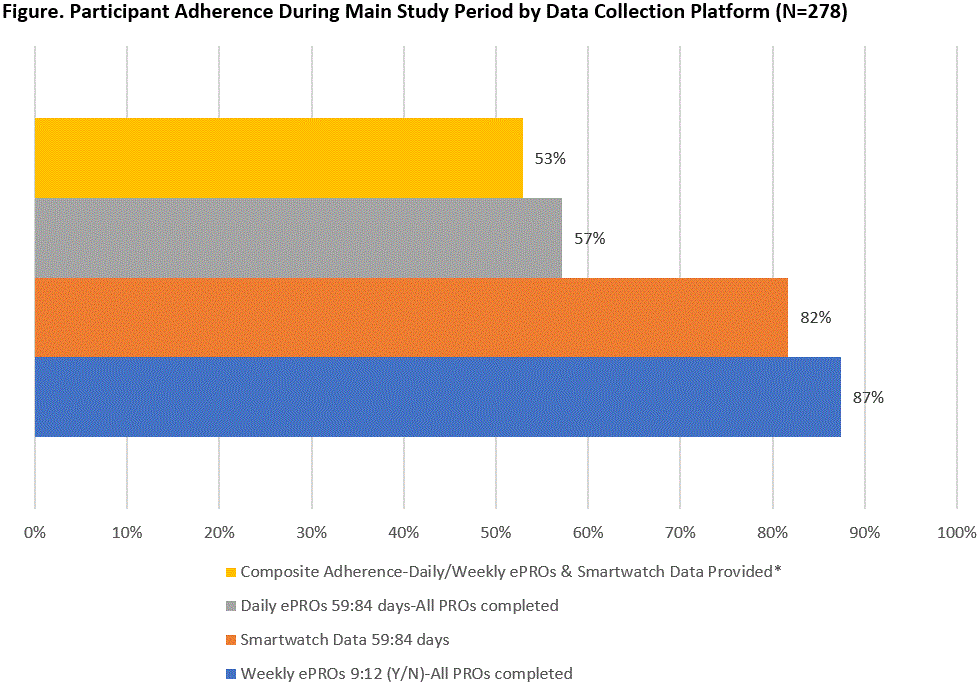
Results: As of 4/2020, and referent to the 470 pts expressing initial interest, 278 (59.1%) completed the Lead-In and qualified for the Main Study. Of the 278 pts enrolled, 91.7% female, mean (SD) age 50.2 (11.1), 9.4 (10.1) years since RA diagnosis (Table 1). Those qualifying for the Main Study were more likely to be currently employed (55.4% vs. 39.6%, p< 0.01) and on biologic monotherapy (63.3% vs. 49.5%, p< 0.01). Over the 84-day Main Study period, the proportion of pts meeting the definition of adherence to protocol-specified data collection was lowest (57%) for daily ePRO data capture, highest for weekly ePRO data (87%), and intermediate (82%) for smartwatch data. A total of 147 (53%) of pts met composite adherence (Figure), while 27/278 met neither smartwatch/PRO adherence, 80/278 met smartwatch adherence but not PRO adherence, and 24/278 did not meet smartwatch adherence but met PRO adherence. Pts experiencing high levels of pain and low levels of physical function at Lead-In were more likely to complete ePROs but not adhere to smartwatch use when they advanced to the Main Study period (Table 2).
Conclusion: Compared to other digital health RA studies, a short lead-in period appears useful to identify pts likely to engage in a longitudinal digital health study collecting data on a mobile app and was associated with subsequent pt adherence, and this adherence may vary by data collection platform. RWE studies involving passive data collection in RA require pt-centric implementation and design to minimize pt burden, promote longitudinal engagement and maximize adherence.
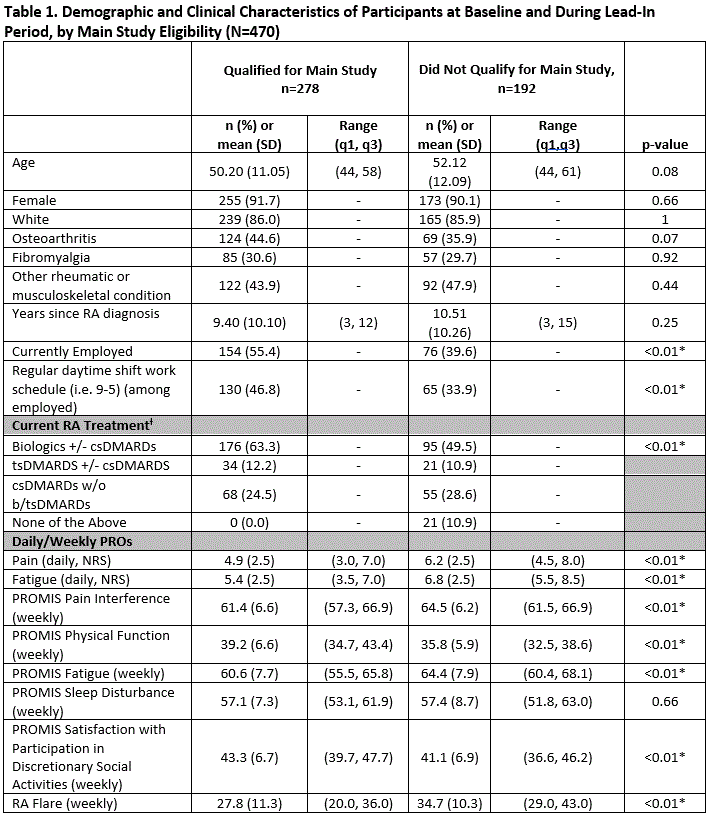 *Statistical significance between groups of pts who qualified and did not qualify for Main Study, p < 0.05; t tests were performed for continuous variables and chi square tests for categorical variables; p values are nominal in nature and should be interpreted in an exploratory manner ƚ DMARDs = disease modifying antirheumatic drugs csDMARDs = conventional synthetic DMARDs (e.g. methotrexate, sulfasalazine) bDMARDs = biologics or biologic DMARDs (e.g. TNFi, IL-6, IL-7) tsDMARDs = targeted synthetic DMARDs (e.g. JAKi) None of the above = on NSAIDs or corticosteroids, but no DMARD or cs/b/tsDMARD
*Statistical significance between groups of pts who qualified and did not qualify for Main Study, p < 0.05; t tests were performed for continuous variables and chi square tests for categorical variables; p values are nominal in nature and should be interpreted in an exploratory manner ƚ DMARDs = disease modifying antirheumatic drugs csDMARDs = conventional synthetic DMARDs (e.g. methotrexate, sulfasalazine) bDMARDs = biologics or biologic DMARDs (e.g. TNFi, IL-6, IL-7) tsDMARDs = targeted synthetic DMARDs (e.g. JAKi) None of the above = on NSAIDs or corticosteroids, but no DMARD or cs/b/tsDMARD
 *Statistical significance between groups of pts who met and did not meet composite adherence in Main Study, p < 0.05; t tests were performed for continuous variables and chi square tests for categorical variables; p values are nominal in nature and should be interpreted in an exploratory manner ƚ DMARDs = disease modifying antirheumatic drugs csDMARDs = conventional synthetic DMARDs (e.g. methotrexate, sulfasalazine) bDMARDs = biologics or biologic DMARDs (e.g. TNFi, IL-6, IL-7) tsDMARDs = targeted synthetic DMARDs (e.g. JAKi)
*Statistical significance between groups of pts who met and did not meet composite adherence in Main Study, p < 0.05; t tests were performed for continuous variables and chi square tests for categorical variables; p values are nominal in nature and should be interpreted in an exploratory manner ƚ DMARDs = disease modifying antirheumatic drugs csDMARDs = conventional synthetic DMARDs (e.g. methotrexate, sulfasalazine) bDMARDs = biologics or biologic DMARDs (e.g. TNFi, IL-6, IL-7) tsDMARDs = targeted synthetic DMARDs (e.g. JAKi)
To cite this abstract in AMA style:
Nowell W, Curtis J, Zhao H, Xie F, Stradford L, Curtis D, Gavigan K, Boles J, Owensby J, Clinton C, Lipkovich I, Venkatachalam S, Nolot S, Haynes V. Participant Engagement and Adherence in an ArthritisPower Real-World Study to Capture Smartwatch and Patient-Reported Outcome Data Among Rheumatoid Arthritis Patients [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/participant-engagement-and-adherence-in-an-arthritispower-real-world-study-to-capture-smartwatch-and-patient-reported-outcome-data-among-rheumatoid-arthritis-patients/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/participant-engagement-and-adherence-in-an-arthritispower-real-world-study-to-capture-smartwatch-and-patient-reported-outcome-data-among-rheumatoid-arthritis-patients/