Session Information
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: End-stage knee osteoarthritis (OA) is often managed with opioids and total knee replacement (TKR). Studies ascertaining opioid use via record review are conflicting as to whether opioid users experience similar pain reduction post TKR compared to non-users. We used claims data to identify opioid prescriptions and analyzed pain response after TKR among naïve, intermittent and continuous opioid users.
Methods: We used Medicare claims data (Parts A/B/D 2010-2014) linked to data from the FORCE-TJR, a research cohort of U.S. patients receiving total joint replacement who completed the Knee Injury and Osteoarthritis Outcome Score (KOOS) at baseline and follow-up. Included patients were ≥ 65 years old, received TKR, and had continuous enrollment in Medicare for the 360 days prior to TKR. We assessed for opioid dispensation in each of the 12 months leading up to TKR. We categorized pre-baseline opioid use as follows, naïve: patients with no opioid prescription, intermittent: those with opioid prescription ≥1 and < 12 months, continuous: patients with a prescription in all 12 months. KOOS Pain (0-100, 100 best) was measured at baseline and at 6 and 12-months post-TKR. We compared post-TKR KOOS Pain at 6 and 12 months among the opioid use groups. We then developed linear regression models to ascertain the association between opioid use and KOOS Pain at 12-months follow up and change in KOOS Pain from baseline to 12-months. For patients not providing 12-month KOOS scores, 6-month data were used. We considered the following covariates baseline KOOS Pain, age, sex, race, body mass index, mental component summary (MCS), comorbidity index, medication use (NSAIDs, COX-2 inhibitors, gabapentin and pregabalin), and indicators of widespread pain- back pain, non-operative knee pain, and neuropathic pain.
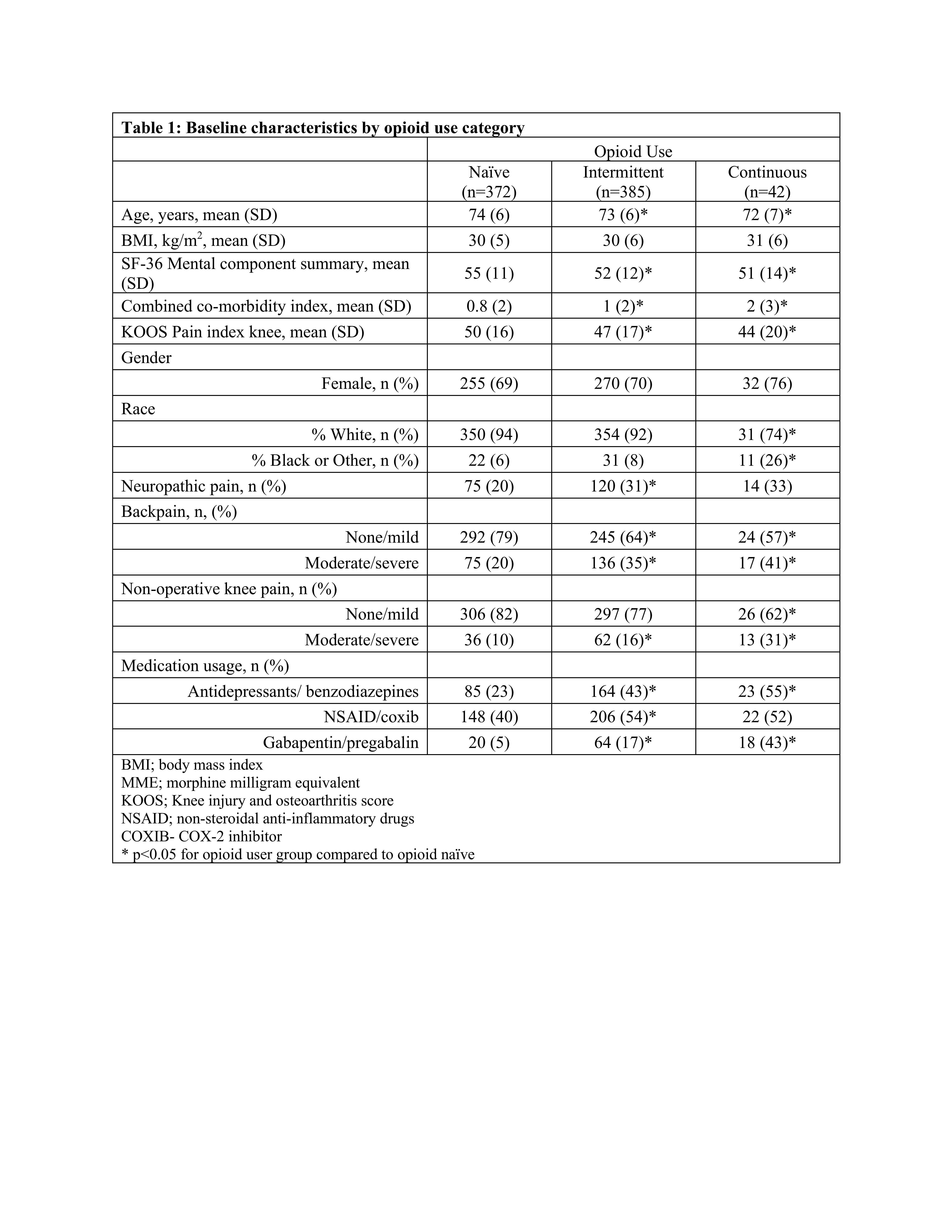
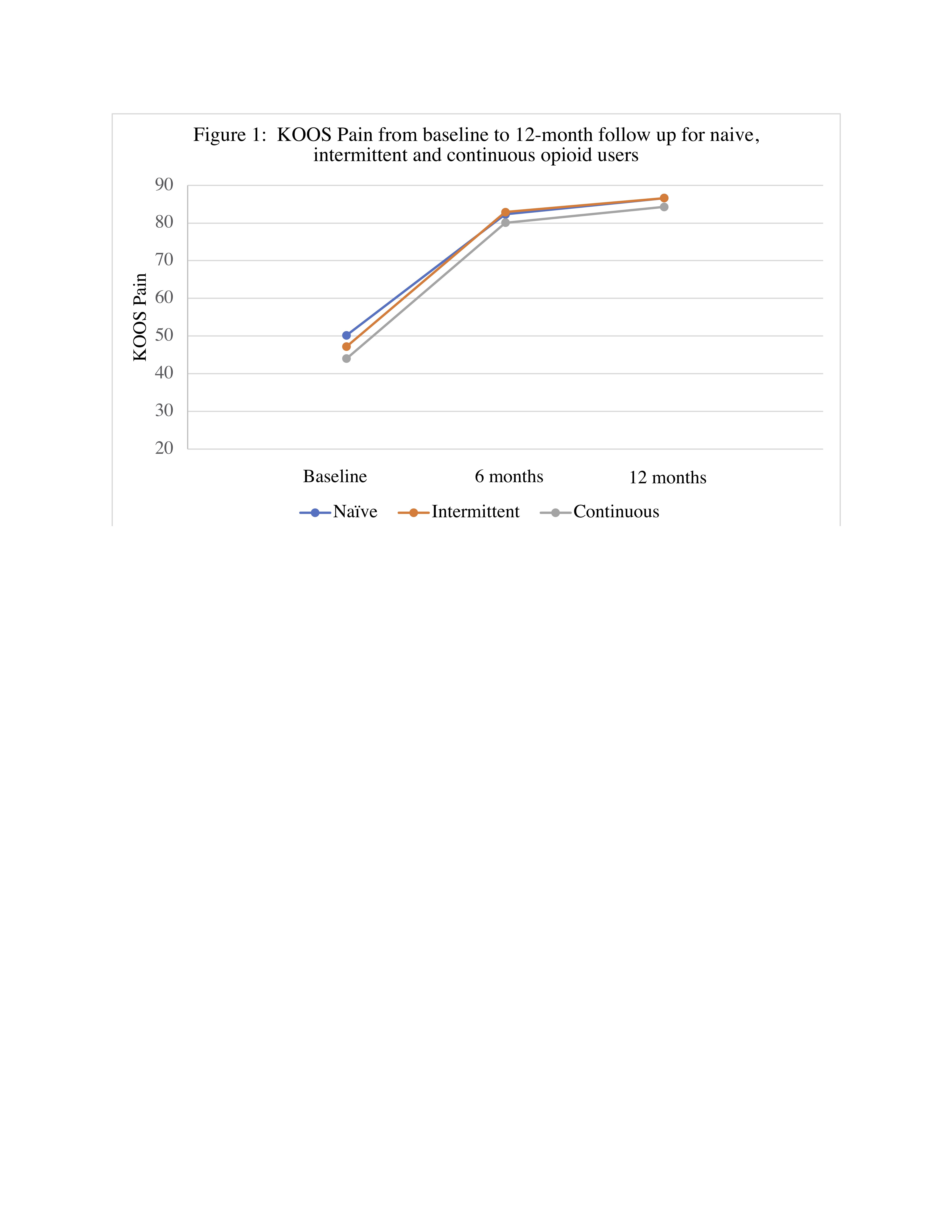
Results: We included 799 patients; 372 were opioid naïve, 385 intermittent users and 42 continuous users. Mean age was 72-73 years and 69-76% were female. Continuous (44±20 p=0.04) and intermittent (47±17 p=0.03) users reported worse KOOS Pain at baseline compared to opioid naïve (50±16) patients. Continuous and intermittent users had more comorbidities and reported greater pain in the back and other knee (Table 1). There were no statistically significant differences in KOOS Pain between opioid users and non-users at 6 and 12-month follow-up (Figure 1). Unadjusted KOOS Pain at 12mo follow up and change in KOOS Pain was similar across the opioid use categories with 85 (∆=35) for naïve patients, 86 (∆=38) for intermittent users and 82 (∆=38) for continuous users. Adjusting for baseline KOOS Pain, and further for demographics, MCS, comorbidity index, medication use and indicators of widespread pain attenuated the difference in both KOOS Pain and change in KOOS Pain across the opioid use categories (Table 2). The differences among the opioid use groups in both the unadjusted and adjusted models are small (effect size < 0.26) and unlikely to be clinically meaningful.
Conclusion: Opioid users, whether intermittent or continuous, achieved similar improvement in knee pain post TKR as opioid naïve patients. This suggests that baseline opioid use alone does not predict which patients will have symptomatic benefit from TKR.
To cite this abstract in AMA style:
MacFarlane L, Jin Y, Lee Y, Lii J, Katz J, Franklin P, Kim S. Pain Reduction Post Total Knee Replacement in Opioid Users and Non-users [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/pain-reduction-post-total-knee-replacement-in-opioid-users-and-non-users/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pain-reduction-post-total-knee-replacement-in-opioid-users-and-non-users/