Session Information
Session Type: Abstract Session
Session Time: 5:00PM-5:50PM
Background/Purpose: Despite the known high inter- and intra-patient variability in analgesic responses in chronic pain, most interventional pain studies rely on single time point pain reports and do not take in consideration the dynamic effects of daily pain on the response to treatment. In this study we examined the between- and within-subject variability in pain ratings and their value as a predictor of treatment response.
Methods: Three hundred and eighty-nine osteoarthritis patients (OA) enrolled as part of a clinical trial (NCT00809783) were randomized in two groups: Naproxen 500 mg twice a day (NAP; n=113) and Placebo (PBO; n=276). All participants were to complete daily pain intensity diaries using a numeric rating scale (NRS; 0 to 10 points) for a period of 127 days (15 days before + 112 days during treatment). A logistic regression analysis of responders (30% pain improvement) and a linear longitudinal model were performed to determine whether the pre-treatment pain variation (standard deviation of pain, SD) was associated with treatment response and decreases in pain intensity.
Results: Longitudinal pain decrease was observed over time (NRS SD) with both treatments (p< 0.001); pain variability (PV) within subjects was stable over time (Pearson’s R, pre/post treatment SD: PBO r= 0.37, p< 0.00; NAP=0.21 p=0.03). Pre-treatment pain variability significantly predicted response after 4, 8, 12 and 16 weeks (Table 1, p< 0.05). Next, we investigated the treatment effects subclassifying treatment groups based on their pain variability. The high variability NAP subgroup presented significantly larger improvement in pain than the low variability subgroup (p< 0.001), and a similar trend was observed for the placebo group (p=0.09) (Figure 1).
Conclusion: Pain variability in patients with OA seems to be stable over time and independent of mean pain reduction. Pre-treatment higher pain variability associates both with categorical response to treatment (30% recovery), and linear pain reduction, thus constituting a marker of treatment response and a possible important effect to control for in OA clinical trials.
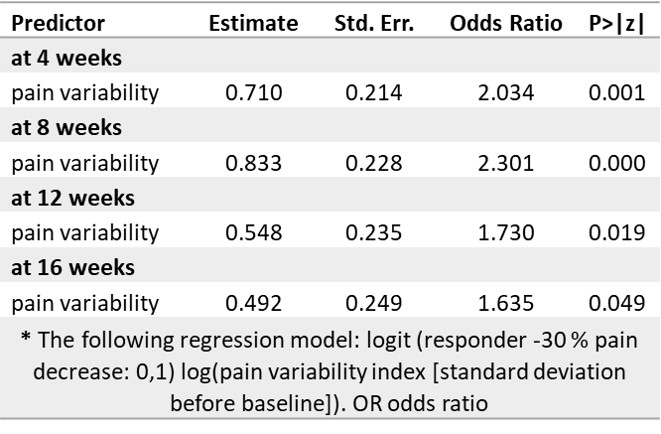 Table 1. Results of logistic regression analysis of clinical pain responders*
Table 1. Results of logistic regression analysis of clinical pain responders*
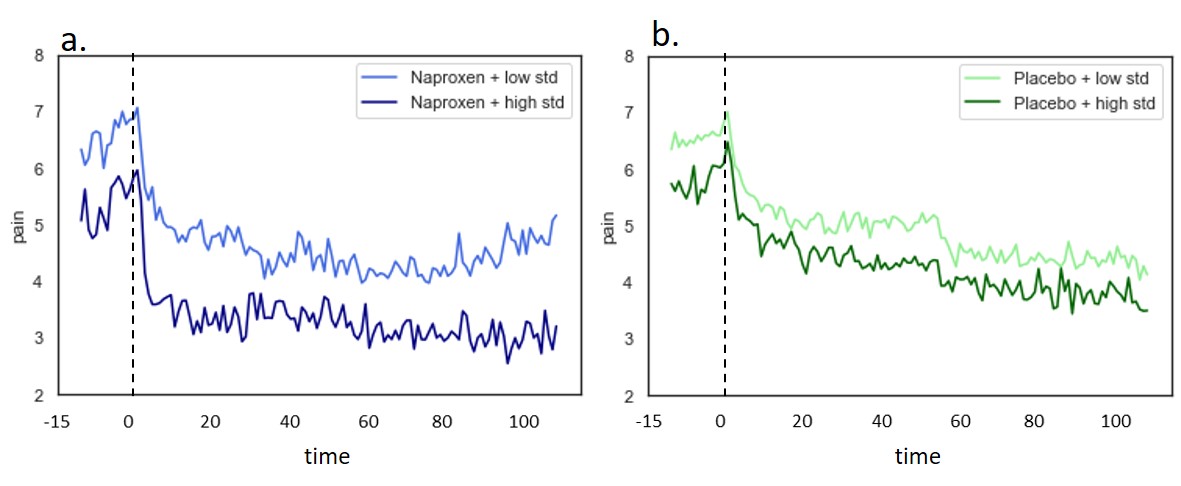 Figure 1: Longitudinal daily pain decreases over time. a. There is significant difference in pain decrease over time between high and low Naproxen groups (p < 0.001). b. There is a significant decrease of pain over time, but not significant difference between high and low Placebo groups (p=0.09).
Figure 1: Longitudinal daily pain decreases over time. a. There is significant difference in pain decrease over time between high and low Naproxen groups (p < 0.001). b. There is a significant decrease of pain over time, but not significant difference between high and low Placebo groups (p=0.09).
To cite this abstract in AMA style:
Pinto C, Barroso J, Schnitzer T. Pain Rating Variability and Response to Treatment in Osteoarthritis Clinical Trials [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/pain-rating-variability-and-response-to-treatment-in-osteoarthritis-clinical-trials/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pain-rating-variability-and-response-to-treatment-in-osteoarthritis-clinical-trials/
