Session Information
Date: Sunday, November 7, 2021
Title: Patient Outcomes, Preferences, & Attitudes Poster II: Measurements (0739–0763)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: People living with idiopathic inflammatory myositis (IIM) suffer impairments in health-related quality of life (HRQL), especially in the domains of pain interference, fatigue, and physical function. In 2021, the OMERACT Myositis Working Group 2021 Survey of PROM Construct Validity and Reliability was developed to evaluate specific patient-reported outcome measures (PROMs). The goal was to assess the measurement properties of construct validity and test-retest reliability for the Patient Reported Outcome Information System (PROMIS) Pain Interference 6a v1.0, Fatigue 7a v1.0, and Physical Function 8b v2.0 instruments.
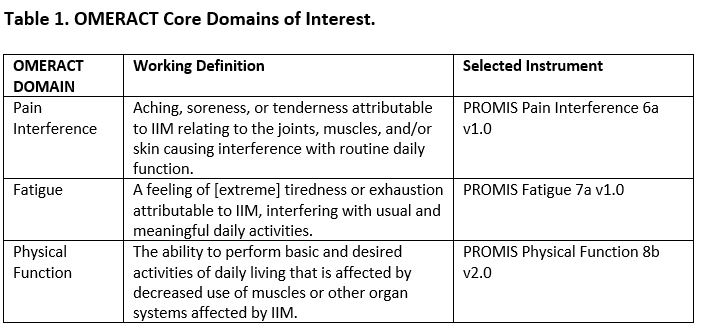
Methods: Formalized definitions of each domain were developed per methodological guidance from the OMERACT Instrument Selection Filter 2.2 (Table 1). PROMs for each domain of interest were deployed via Redcap to adult patients with confirmed IIM at a large tertiary care referral site (Baltimore, MD). Patient demographics and PROMIS v1.0 4a anxiety, depression, sleep disturbance, and social participation questionnaires were also administered, and identical questionnaires were given 7 days later. In addition, data from Sweden, Australia, South Korea, The Netherlands and the USA that was collected as part of the OMERACT Myositis Working Group 2019 Survey of PROM Content Validity and Feasibility was used which additionally included the Pain Disability Index (PDI), International Physical Activity Questionnaire (IPAQ), and Myositis Activities Profile (MAP). Raw PROMIS scores were converted to T-score measures and normalized to a score of 50 with a standard deviation of 10; higher scores indicate more of the concept being measured. Test/Retest reliability was calculated using intraclass correlation coefficients (ICC) and correlation matrices were constructed. Construct validity was determined via formulation of a priori hypotheses generated by working group members (Table 2); >75% member agreement was required for each hypothesis. Pearson’s correlation was calculated for each construct pair.
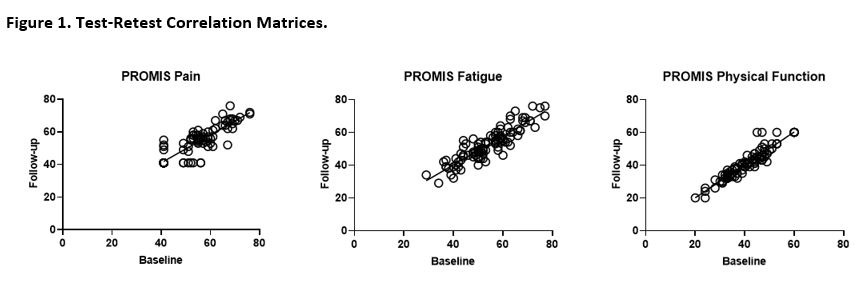
Results: Domain definitions were formalized based on patient/expert consensus (Table 1). There were 287 adult participants with IIM who completed the baseline 2021 survey and 97 participants who completed the retest; the number of participants who completed the 2019 survey as relevant to this exercise varied (Table 2). Participants from the 2021 baseline survey were 76% female with a mean (SD) age of 60 (12). Test-retest reliability was high for pain interference, fatigue, and physical function with an ICC (95% CI) of 0.878 (0.823-0.917), 0.886 (0.822-0.925), and 0.943 (0.917-0.962), respectively (Figure 1). For the construct validity exercise, member consensus was met for 12 of 13 a priori hypotheses. Hypotheses were correct for 3 out of the 4 available constructs for pain interference, 4 out of 4 constructs for fatigue, and 4 of 5 constructs for physical function (Table 2).
Conclusion: Both test-retest reliability and construct validity are high for pain interference, fatigue, and physical function when used in this IIM population. Further longitudinal validation is ongoing in Sweden, Australia, South Korea, The Netherlands and the USA.
To cite this abstract in AMA style:
DiRenzo D, de Groot I, Alexanderson H, Bingham C, Lundberg I, Needham M, Park J, Regardt M, Sarver C, Saygin D, Song Y, Maxwell L, Beaton D, Christopher-Stine L, Mecoli C. Pain Interference, Fatigue, Physical Function as Outcome Measures in Adult Myositis: Updates on the Validation Process by the OMERACT Myositis Working Group [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/pain-interference-fatigue-physical-function-as-outcome-measures-in-adult-myositis-updates-on-the-validation-process-by-the-omeract-myositis-working-group/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pain-interference-fatigue-physical-function-as-outcome-measures-in-adult-myositis-updates-on-the-validation-process-by-the-omeract-myositis-working-group/