Session Information
Date: Saturday, November 16, 2024
Title: SLE – Animal Models Poster
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Skin involvement is the most common organ manifestation in systemic lupus erythematosus (SLE), and exacerbation of cutaneous lupus (CLE) can precede systemic disease flares. Keratinocyte-derived interferon kappa (IFN-κ) is an important mediator of photosensitive responses in SLE and is chronically overexpressed in SLE non-lesional skin. However, the effects of a type I IFN-rich epidermis on immune system activation after UV is not well understood and might be crucial to understand UV-driven disease flares in SLE. Here, we explored responses to chronic UV exposure in vivo using a mouse model of overexpression of epidermal Ifnk.
Methods: We compared female 12-week-old BALB/c mice that overexpress Ifnk in the epidermis (TG) with BALB/c wild-type mice (WT) and assessed local and systemic immune responses at baseline and after UVB exposure. Mice were shaved, then irradiated with 200mJ/cm2 for 3 days per week for 2 weeks and observed for 2 weeks after irradiation. Cutaneous lesion size, proteinuria, and peripheral blood count were measured. Skin-draining lymph nodes were examined for T cell activation. Flow cytometry on splenocytes was performed to phenotype myeloid, T cells, and B cells.
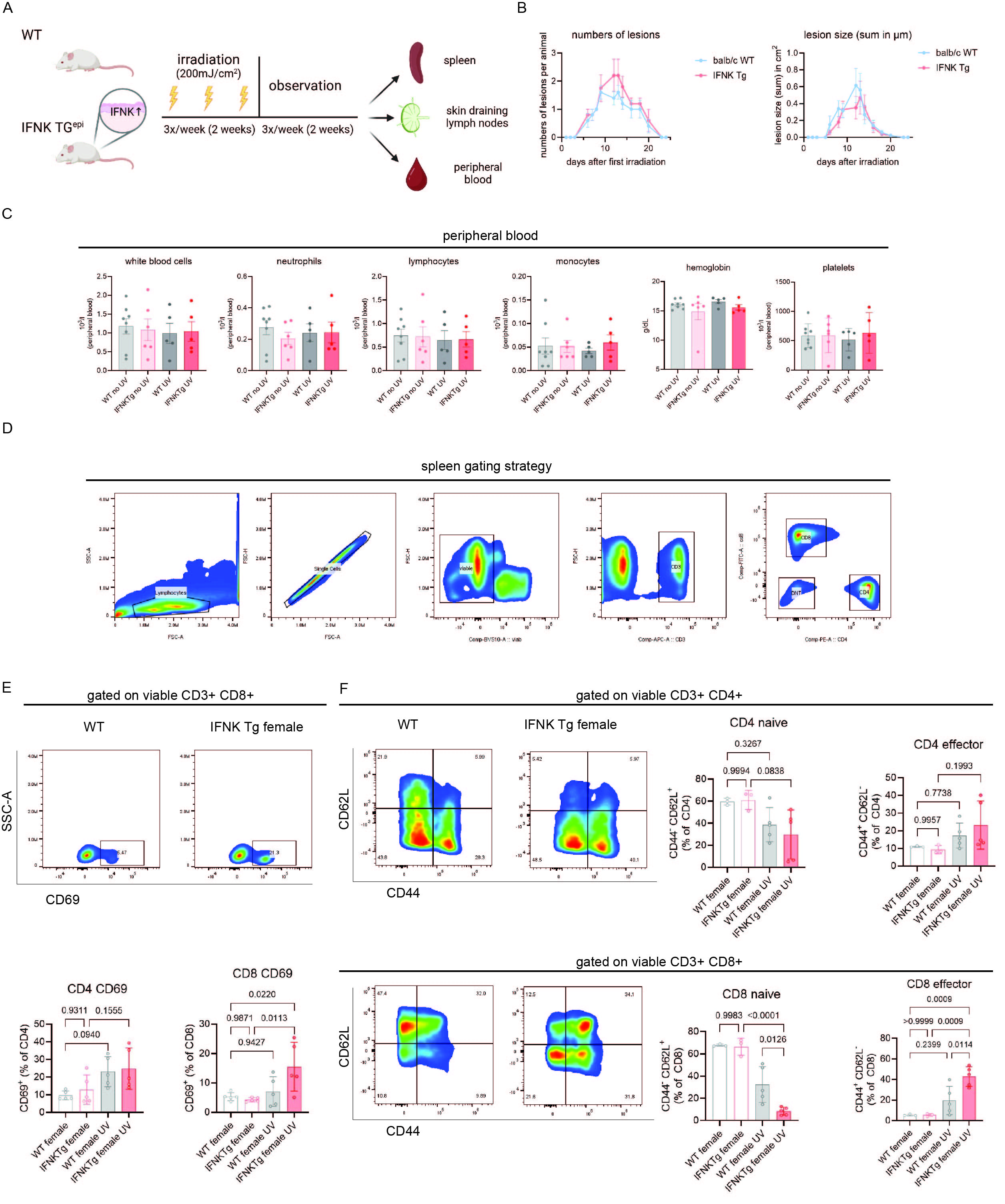
Results: At baseline, both WT and TG mice were without skin lesions, proteinuria, or abnormalities in peripheral blood counts. After UVB exposure, both TG and WT mice developed cutaneous lesions that healed completely during the observation period post-irradiation. Peripheral blood counts revealed slight increases in monocytes in TG compared to WT mice after UVB exposure. Strikingly, TG mice showed higher T cell activation (CD69) in the spleen than WT post-UVB treatment. This was accompanied by a significant shift of naïve CD8+ T cells (CD62L+CD44–) towards effector CD8+ T cells (CD62L–CD44+) in TG compared to WT mice post-UVB treatment. B cell phenotyping revealed a similar abundance of naïve and switched GL7+ and plasma cells in both strains at baseline and post-UV. Similar increases in CD8+ T cell activation were seen in the draining lymph nodes in UV-exposed TG vs. WT mice, suggesting that CD8+T cell activation may spread from local to systemic in the context of chronic epidermal IFNs.
Conclusion: Our results identify a switch in the regulation of systemic T cell responses in the context of chronic epidermal type I IFN production. Further exploration will address which IFN-κ-induced factors are regulating these responses and further delineate the link between epidermal inflammation and disease flares in systemic lupus.
A) Experimental approach. Female WT and mice with overexpression of epidermal IFNk (IFNK Tg) were irradiated three days per week (UVB 300mJ/cm2) for two weeks and then observed for two more weeks. Peripheral blood, skin draining lymph nodes and spleens were harvested for immunophenotyping. B) Time course of number and size of skin lesions in WT and IFNK Tg mice after UVB irradiation (n=5). C) Peripheral blood counts before and after UV in female WT and IFNK Tg mice. D) Spleen gating strategy for T cells. E) Representative flow cytometry plots of splenic CD69+ CD8+ T cells and quantification of splenic CD69+ CD4+/CD8+ T cells in WT and IFNK Tg mice after UVB exposure. F) Representative flow cytometry plots and quantification of naive (CD62L+CD44-) and effector memory (CD62L-CD44+) CD4+/CD8+ T cells in WT and IFNK Tg mice before and after UV irradiation. Two-way ANOVA followed by Šídák’s multiple comparisons test, mean and SEM.
To cite this abstract in AMA style:
Klein B, McNeely K, Colesa D, Gao Y, Nguyen N, Gudjonsson J, Kahlenberg J. Overexpression of Epidermal Ifnk Promotes Systemic CD8+ T Cell Maturation After UV-exposure [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/overexpression-of-epidermal-ifnk-promotes-systemic-cd8-t-cell-maturation-after-uv-exposure/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/overexpression-of-epidermal-ifnk-promotes-systemic-cd8-t-cell-maturation-after-uv-exposure/