Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Immune checkpoint inhibitors (ICI) increase one-year survival in metastatic non-small cell lung cancer (mNSCLC) from 49% to 69% with chemotherapy-based regimens. Patients with rheumatoid arthritis (RA) have a 60% higher risk of lung cancer than the general population but were not included in seminal ICI trials. The goal of this study was to compare overall survival (OS) in ICI-treated patients with mNSCLC with pre-existing RA versus without RA.
Methods: We used a curated Medicare claims dataset that consist of a 100% sample of patients with RA. ICI use was identified using Healthcare Common Procedure Coding System (HCPCS) codes. Patients with mNSCLC were defined by having two claims at least two months apart associated with an ICD-9 162.9 or ICD-10 C34.X diagnosis code for malignant neoplasm of lung and bronchus. We limited the cohort to patients who initiated ICI between 2015-2019 because ICI was only approved for mNSCLC during this period, and not for earlier stages or other types of lung cancer. RA was defined as having two claims associated with an ICD-9-CM or ICD-10-CM diagnosis code for RA. ICI-treated non-RA comparator patients were identified in the Medicare 5% sample. Descriptive statistics were calculated for each cohort and for overall survival. Kaplan Meier curves and adjusted log logistic survival models were created to measure overall survival, anchored at the time of first ICI initiation. Patients were followed through 12/31/2019 and were censored at time of death or last recorded visit in the database.
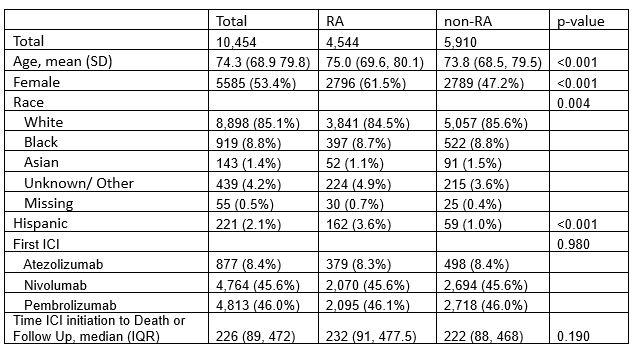
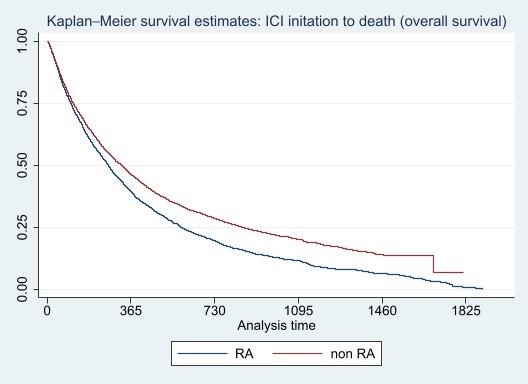
Results: A total of 10454 ICI-treated patients with mNSCLC were included in the analysis; 4,544 with RA (100% sample) and 5910 without RA (5% sample). Overall mean age was 74.3 (SD 8.22) and 85% were White and non-Hispanic (Table 1). Patients with RA were more likely to be female, White and Hispanic than non-RA patients. Median time to death from first ICI initiation was 180 days (IQR 73,370) and 70% died (N=7,305). Figure 1 displays overall survival for ICI-treated mNSCLC patients with RA compared to those without RA.
Conclusion: Patients with pre-existing RA who develop lung cancer and initiate ICI for mNSCLC appear to have significantly worse overall survival compared to patients without RA. Further refinement of the cohort and further analyses of this dataset, including modeling, that incorporate prior lines of cancer therapy, receipt of concomitant chemotherapy and treatment with RA disease modifying antirheumatic drugs will be needed to confirm these findings.
To cite this abstract in AMA style:
Jannat-Khah D, Curtis J, Xie F, Saxena A, Bass A. Overall Survival in Patients with versus Without Rheumatoid Arthritis Initiating Immune Checkpoint Inhibitors for Metastatic Non Small Cell Lung Cancer [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/overall-survival-in-patients-with-versus-without-rheumatoid-arthritis-initiating-immune-checkpoint-inhibitors-for-metastatic-non-small-cell-lung-cancer/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/overall-survival-in-patients-with-versus-without-rheumatoid-arthritis-initiating-immune-checkpoint-inhibitors-for-metastatic-non-small-cell-lung-cancer/