Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Use of high dose corticosteroids in systemic sclerosis (SSc) is considered a significant risk factor for development of scleroderma renal crisis (SRC). Intravenous (IV) methylprednisolone is commonly used for prevention of infusion reactions and drug forming antibodies with infusion biologics. With increasing use of biologic medications such as rituximab (RTX), we studied whether intermittent high dose IV corticosteroid premedication is associated with development of SRC in SSc patients.
Methods: We performed a retrospective observational study of EMR data from 155 SSc patients seen long-term in Rheumatology Clinic at University of New Mexico Hospital between January 2009 and December 2023. R statistical software was used to compare outcomes in SSc patients who received premedication IV methylprednisolone prior to infusion of a biologic against a control group of SSc patients who had not received any IV corticosteroid doses.
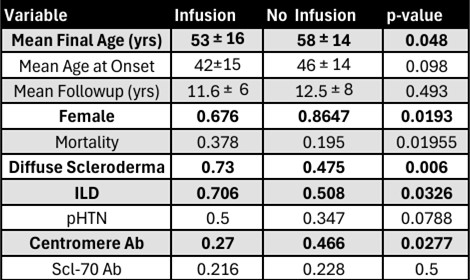
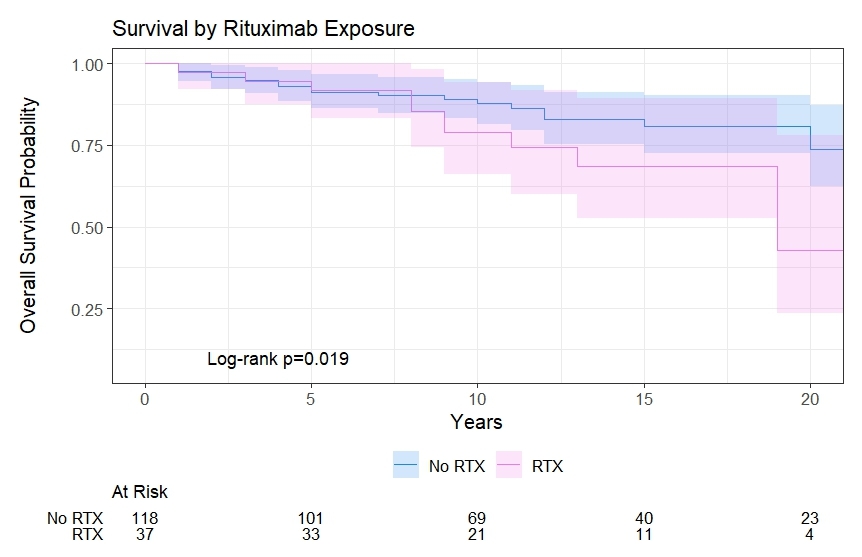
Results: 39 of 155 SSc patients received a methylprednisolone IV infusion. 37 out of the 39 infusion patients received RTX therapy. The infusion and no-infusion groups were similar in age of SSc onset, and length of follow up (Figure 1). However, the infusion group was at particularly high risk for SRC: 1) 100% (39/39) received corticosteroids, 2) 76% (28/37) were diffuse SSc, 3), 73% (27/37) were anti-centromere Ab negative, 4) 22% (8/37) were anti-RNAP III positive, and 5) 22% (8/37) anti-topoisomerase ab positive. No patients in the control group developed SRC during the observation period. In the infusion group, one patient developed SRC prior to first infusion, but across a total of 827 infusions with a mean of 21±23 infusions per patient and a mean IV steroid exposure of 135mg±66mg prednisone equivalents per infusion (mean 2983mg±5198mg prednisone equivalents per patient over the study period), none of the 39 total infusion patients developed SRC during or after infusion therapy. Patients receiving RTX infusion had significantly higher overall all-cause mortality (HR 2.23, 95% CI 1.14-4.37, p=0.020) but had similar 5-year (92% vs 91%) survival rates (Figure 2).
Conclusion: The present data suggest that intermittent high dose IV methylprednisolone premedication is not associated with development of new or relapsing SRC compared to patients not on infusion biologic therapy even in a cohort with very significant baseline SRC risk factors. While all cause mortality was increased in the RTX infusion group, this group had also had a significantly higher proportion of males, Interstitial Lung Disease (ILD), and Diffuse Cutaneous Scleroderma, all factors that have been independently associated with increased mortality in SSc (Figure 3). While further research is needed to evaluate risk of SRC with different classes of infusion medication, the risk of non-SCR corticosteroid related toxicities, and the magnitude of benefit for IV methylprednisolone premedication in this population, our data suggest that intermittent IV corticosteroid premedication for biologic infusions appears broadly safe in SSc patients.
To cite this abstract in AMA style:
Adarsh V, Fatma d, Akpan E, Keller M, Muruganandam M, Emil N, O'Sullivan F, Fields R, sibbitt w. Outcomes of Intermittent Intravenous Steroid Use During Infusion Therapy in Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/outcomes-of-intermittent-intravenous-steroid-use-during-infusion-therapy-in-systemic-sclerosis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/outcomes-of-intermittent-intravenous-steroid-use-during-infusion-therapy-in-systemic-sclerosis/