Session Information
Date: Tuesday, November 9, 2021
Title: Systemic Sclerosis & Related Disorders – Clinical Poster III (1836–1861)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Autologous haematopoietic stem cell transplantation (SCT) has emerged as an effective treatment for patients with severe diffuse systemic sclerosis (dcSSc) based upon the results of two large clinical trials demonstrating long term benefit for survival, skin and lung involvement and quality of life. Over the past two decades routine management of dcSSc has improved and immunosuppressive drugs are increasingly administered in the early course of the disease. These developments may have improved survival of cases that would have been eligible for SCT trials compared with historically predicted outcomes. The aim of this study was to explore outcomes in a cohort of dcSSc patients fulfilling eligibility criteria for SCT studies but receiving contemporary standard treatment.
Methods: From a single-centre cohort dcSSc patients (n=636) were selected using the SCT trials’ inclusion criteria (Table 1). Patients meeting the trials’ exclusion criteria were excluded. Overall survival and event free survival (EFS) of patients eligible for one or more of the studies (ASTIS, SCOT or the ongoing UPSIDE trial) were assessed using Kaplan‐Meier survival estimates. Hazard ratios (HR) were calculated using Cox proportional hazards regression analysis. EFS was defined as the time in years from eligibility until the occurrence of death due to any cause or development of major organ damage, defined as severe cardiac involvement or pulmonary fibrosis, scleroderma renal crisis (SRC) or pulmonary hypertension (PH).
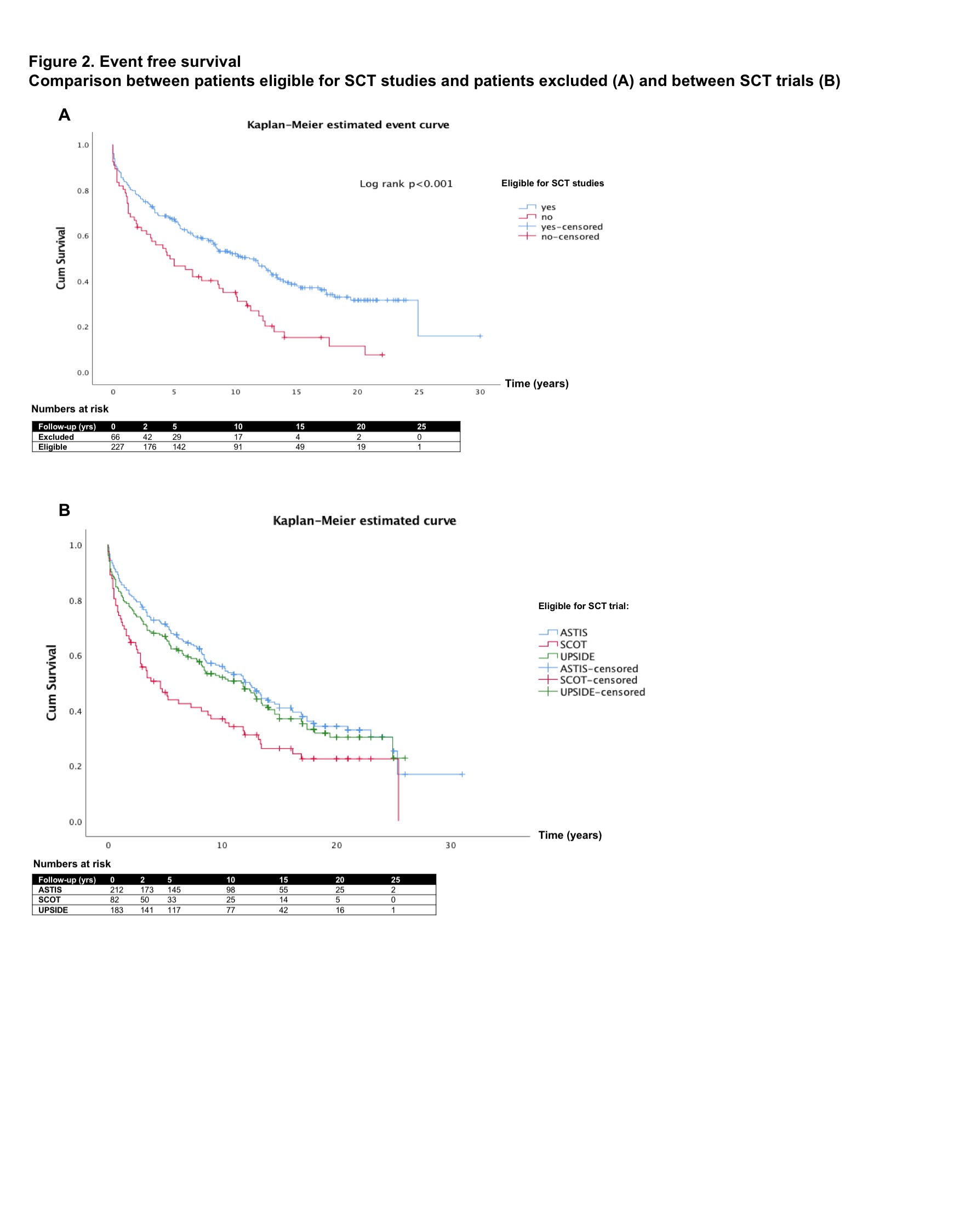
Results: Of the 227 eligible patients, 214 met the inclusion criteria for ASTIS, 82 for SCOT and 185 for the UPSIDE trial. 66 patients were excluded based on age > 65 years, low DLco, pulmonary hypertension or creatinine clearance < 40ml/min. There was overlap between eligible patient groups (Figure 1). The median follow-up time was 12 years (IQR 9). Among eligible patients, 103 (45.4%) died. Survival was 96% at 2-, 88% at 5-, 73% at 10- and 43% at 20 years. Compared to this ‘SCT-eligible’ cohort, those patients who would have been excluded from SCT trials had a worse long-term overall survival (97% at 2-, 77% at 5-, 52% at 10- and 15% at 20 years, log rank p< 0.001). Excluded patients also had a significantly worse EFS (Figure 2A). Differences between the three different trials were observed as well (Figure 2B). Hazard of death was higher in patients with higher age at onset (HR 1.05, p< 0.001), higher ESR at onset (HR 1.01, p=0.025) and males (HR 2.12, p=0.008). Male sex was also an independent risk factors for a serious event (HR 1.97, 95% CI 1.34-2.88, p=0.001). Higher DLco at onset was associated with a lower hazard for an event (HR 0.98, 95% CI 0.97-0.99, p=0.001) in the multivariable Cox regression analysis.
Conclusion: This study demonstrates that SCT inclusion criteria identify patients with poor outcome despite current best practice treatment as long-term outcomes were unfavorable in our cohort compared to SCT in the trials. It shows that SCT trials have overlapping eligibility criteria but may recruit different populations which requires caution when comparing results. Our findings emphasize the need for better treatment strategies for the very poor outcome patients who are excluded from SCT.
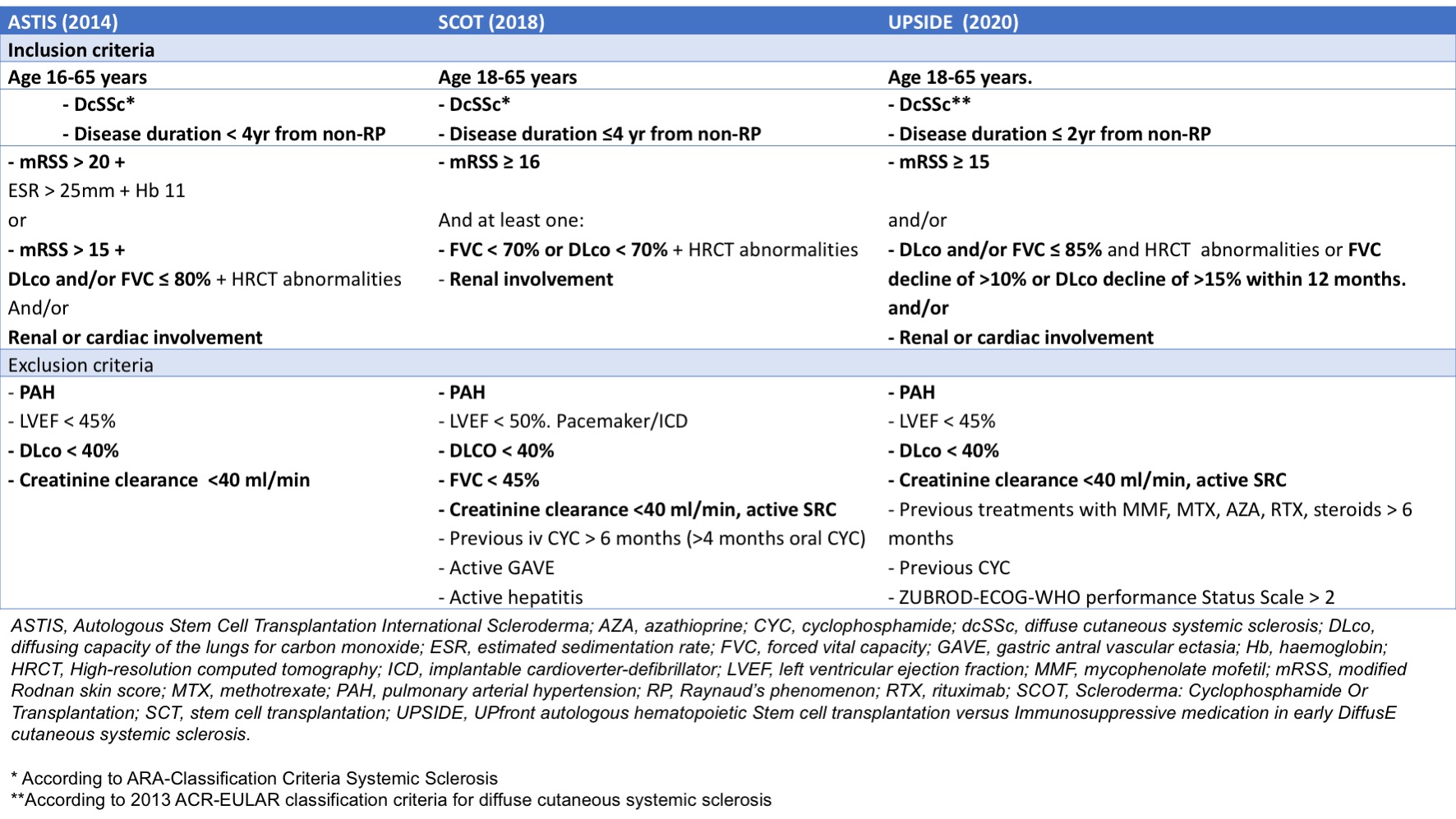 Table 1.jpeg”Table 1. Inclusion criteria from SCT trials. In bold: criteria used to select patients for this study.
Table 1.jpeg”Table 1. Inclusion criteria from SCT trials. In bold: criteria used to select patients for this study.
 Figure 1. Venn diagram showing overlap of patients eligible for stem cell transplantation trials
Figure 1. Venn diagram showing overlap of patients eligible for stem cell transplantation trials
To cite this abstract in AMA style:
Spierings J, Nihtyanova S, Derrett-Smith E, Clark K, van Laar J, Ong V, Denton C. Outcomes Linked to Eligibility for Stem Cell Transplantation Trials in Diffuse Cutaneous Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/outcomes-linked-to-eligibility-for-stem-cell-transplantation-trials-in-diffuse-cutaneous-systemic-sclerosis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/outcomes-linked-to-eligibility-for-stem-cell-transplantation-trials-in-diffuse-cutaneous-systemic-sclerosis/