Session Information
Date: Monday, November 13, 2023
Title: Abstracts: Systemic Sclerosis & Related Disorders II: Clinical Research
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: Autologous hematological stem cell transplantation (AHSCT) is a grade A therapy for early diffuse progressive systemic sclerosis (SSc), that has been validated in three randomized controlled trials (RCT) compared to cyclophosphamide (CYC). CYC, however, is no longer considered the gold standard therapy for SSc and does not provide long-term benefit. The efficacy of rituximab on skin and lung involvement in SSc has recently been demonstrated in an RCT, the DESIRES study. The combination of rituximab with mycophenolate mofetil (MMF) is a potential potent regimen for progressive SSc, that has not been evaluated compared to AHSCT. We retrospectively compared the outcomes of SSc patients in our cohort, fulfilling eligibility criteria for AHSCT studies, who received a combination therapy of MMF and rituximab, with patients who underwent AHSCT.
Methods: Repeated longitudinal assessments at baseline and every 6 months for 24 months, including modified Rodnan Skin Score (mRSS), forced vital capacity (FVC), and diffusing capacity of the lung for carbon monoxide (DLCO) values, were compared between groups using a linear mixed model regression. Clinical improvement (CI) was defined as a decrease in mRSS by more than 25% or an increase in FVC by more than 10%. Event-free survival (EFS) was defined as the occurrence of death or the development of persistent major organ failure (heart, lung, kidney). Hazard ratios (HRs) and 95% CIs were calculated by Cox regression.
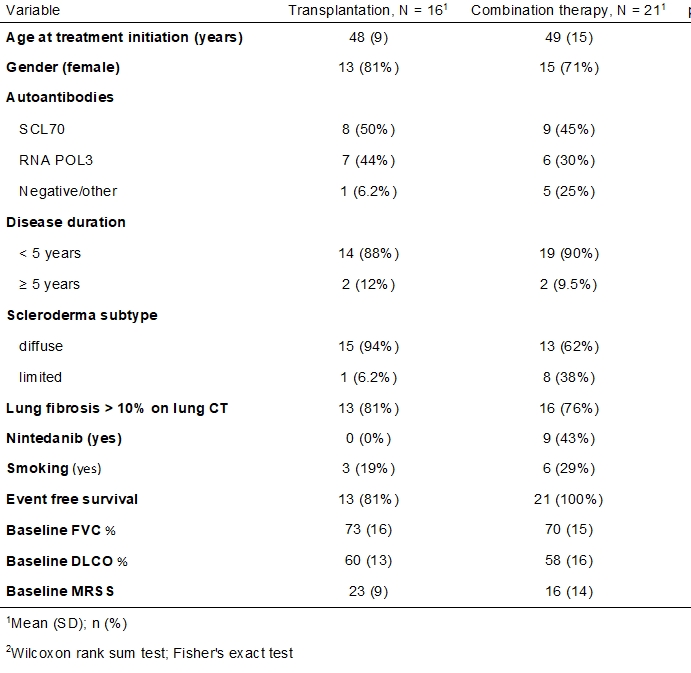
Results: Twenty-one SSc patients in the combination group were compared to sixteen in the AHSCT group. There were no significant differences in the demographic or clinical variables, except for Nintedanib treatment (Table 1). CI at 12 months was seen in 86% of patients in the combination group, compared to 81% in the AHSCT group (p=0.7). The hazard ratio (HR) for EFS at 24 months favored the combination group (HR=0.09, 95% CI 0.009 to 0.9; P =0.04). In both groups, a statistically significant and similar increase in FVC values, but not in DLCO, was demonstrated at each time point during follow-up. The inclusion of Nintedanib usage in the mixed model did not influence these results. In both groups, there was a significant and similar reduction in mRSS at 12 and 24 months compared to baseline (figure 1).
Conclusion: In our cohort, combination therapy of MMF and rituximab compared to AHSCT in SSc patients eligible for AHSCT, resulted in similar skin and lung clinical improvement with a better safety profile after 24 months.
2Wilcoxon rank sum test; Fisher’s exact test
To cite this abstract in AMA style:
keret s, Kaly L, Shouval A, Zuckerman T, Henig I, Awisat A, Rosner I, Rozenbaum M, Boulman N, Molad Y, Dortort Lazar a, Slobodin G, Rimar D. Outcomes in Systemic Sclerosis Patients Treated with Rituximab and Mycophenolate Mofetil Combination Therapy Compared to Autologous Hematological Stem Cell Transplantation [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/outcomes-in-systemic-sclerosis-patients-treated-with-rituximab-and-mycophenolate-mofetil-combination-therapy-compared-to-autologous-hematological-stem-cell-transplantation/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/outcomes-in-systemic-sclerosis-patients-treated-with-rituximab-and-mycophenolate-mofetil-combination-therapy-compared-to-autologous-hematological-stem-cell-transplantation/