Session Information
Date: Saturday, November 6, 2021
Title: Epidemiology & Public Health Poster I: COVID-19 & Vaccination (0084–0117)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Janus kinase inhibitors (JAK-i) offer a potent mode of action to treat rheumatic diseases. Little is known on the course and outcome of SARS-CoV-2 infections in RA patients treated with JAK-i. The aim of this study is to describe the outcome of SARS-CoV-2 infection in RA patients treated with JAK-i in comparison to patients treated with TNF-inhibitors (TNF-i).
Methods: In the German COVID-19 registry for inflammatory rheumatic diseases, the course and outcome of proven SARS-CoV-2 infections are documented by treating rheumatologists. From March 30th, 2020 until April 5th, 2021 a total of 2253 cases was collected. 982 were reported with RA, of which 128 were treated with JAK-i and 190 with TNF-i. Patient characteristics and outcome of the SARS-CoV-2 infection were analysed descriptively. Differences in characteristics were tested by the Pearson Chi-Square-Test.
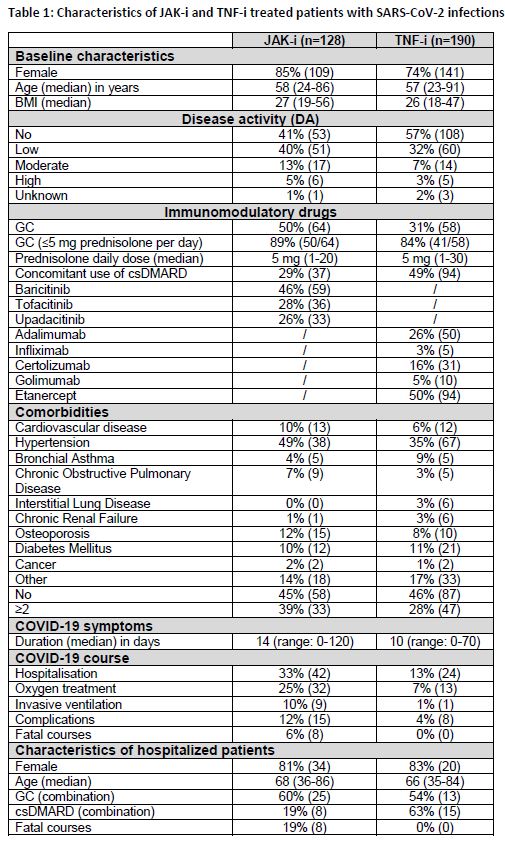
Results: Median age in both treatment groups was comparable (tab.1). Concomitant glucocorticoids (GC) were used in 50% of the JAK-i and 31% of the TNF-i patients (p < 0.001). Additional conventional synthetic (cs) disease-modifying anti-rheumatic drugs (DMARD) were used in 29% of the JAK-i and in 49% of the TNF-i patients (p < 0.001). Moderate and high disease activity before acquisition of the infection was more frequently reported in JAK-i patients than under TNF-i treatment (p=0.042). Comorbidities that were recently found to be associated with a higher risk of hospitalisation and death, namely cardiovascular disease and hypertension were also more frequent in patients with JAK-i. Hospitalisation due to COVID-19 was necessary for 33% of patients treated with JAK-i and 13% of patients treated with TNF-i , and oxygen treatment was required in 25% and 7% of the patients, respectively (p < 0.001). Eight versus none (p < 0.001) fatal cases were reported in the JAK-i versus the TNF-i group.
Conclusion: The higher rate of hospitalisation and death among RA patients treated with JAK-i compared to TNF-i is remarkable. However, this has to be interpreted with caution, since RA patients under JAK-i harboured some differences in potentially confounding RA-related and unrelated factors like disease activity, concomitant use of GCs, and certain comorbidities. To address this, further analyses with adjustments for baseline differences should follow.
To cite this abstract in AMA style:
Hasseli R, Hoyer B, Lorenz H, Pfeil A, Regierer A, Richter J, Schmeiser T, Strangfeld A, Voll R, Krause A, Schulze-Koops H, Specker C, Müller-Ladner U. Outcome of SARS-CoV-2 Infection in Patients with Rheumatoid Arthritis Under Treatment with Janus Kinase Inhibitors Compared to Tumour Necrosis Factor Inhibitors [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/outcome-of-sars-cov-2-infection-in-patients-with-rheumatoid-arthritis-under-treatment-with-janus-kinase-inhibitors-compared-to-tumour-necrosis-factor-inhibitors/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/outcome-of-sars-cov-2-infection-in-patients-with-rheumatoid-arthritis-under-treatment-with-janus-kinase-inhibitors-compared-to-tumour-necrosis-factor-inhibitors/