Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Recent RCTs demonstrated that TNFα inhibition has no effect on pain and MRI-detected synovitis or bone marrow lesions in patients with erosive hand OA. However, the progression of bone erosions was reduced in a subgroup of patients with more clinically swollen distal interphalangeal joints in one RCT. Consequently, it remains possible that TNFα inhibition may have beneficial effects in specific subgroups of patients with a high inflammatory component. We aimed to evaluate the efficacy and safety of a TNFα inhibitor, adalimumab (ADA), in a proof-of-concept study in patients with inflammatory OA of the knee.
Methods: OKINADA was a 52-week, randomized, double-blind, placebo-controlled, parallel-group study done at 11 sites in Canada (NCT02471118). Eligible participants were adults with a diagnosis of OA of the index knee and classified according to American College of Rheumatology criteria, including radiological evidence of OA (Kellgren-Lawrence grades 2 or 3), with clinical signs of knee effusion. Subjects had persistent knee pain of ≥ one month duration with a pain score of ≥ 4 (0-10 NRS) in the index knee at screening and baseline despite conventional treatment with maximum tolerated acetaminophen and/or non-steroidal anti-inflammatory drug. Patients were randomly assigned (1:1) to receive subcutaneous 40 mg ADA every 2 weeks or placebo (PBO) to week 16. All patients then received open label ADA to week 32. Primary endpoint was the Outcome Measures in Rheumatology and Osteoarthritis Research Society International set of responder criteria (OMERACT-OARSI) at week 16. Secondary endpoints included: the Knee Injury and Osteoarthritis Outcome Score (KOOS) for the domains of pain, activities of daily living (ADL), OA symptoms, sport and recreation function (SRF), and knee-related quality of life (QoL), patient global assessment of disease status (PGAD), investigator global assessment of disease status (IGAD), and expanded Target Joint Assessment (TJA) score.
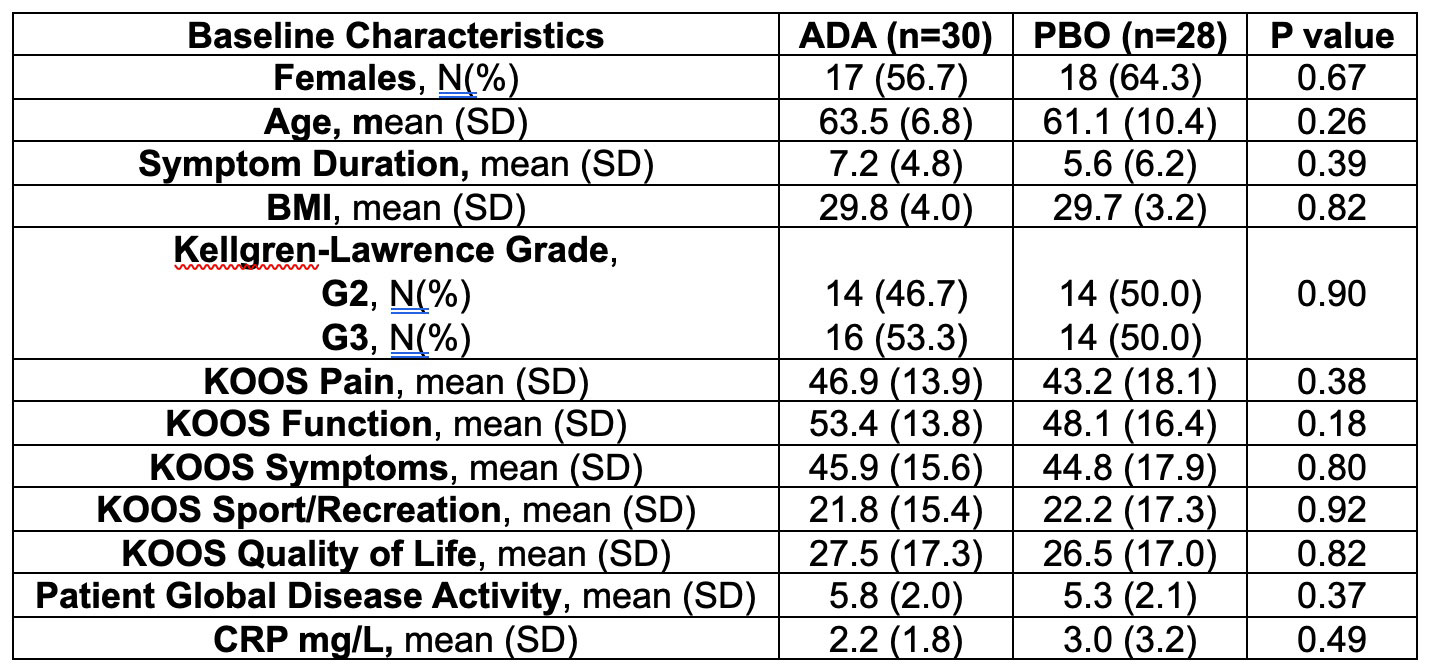
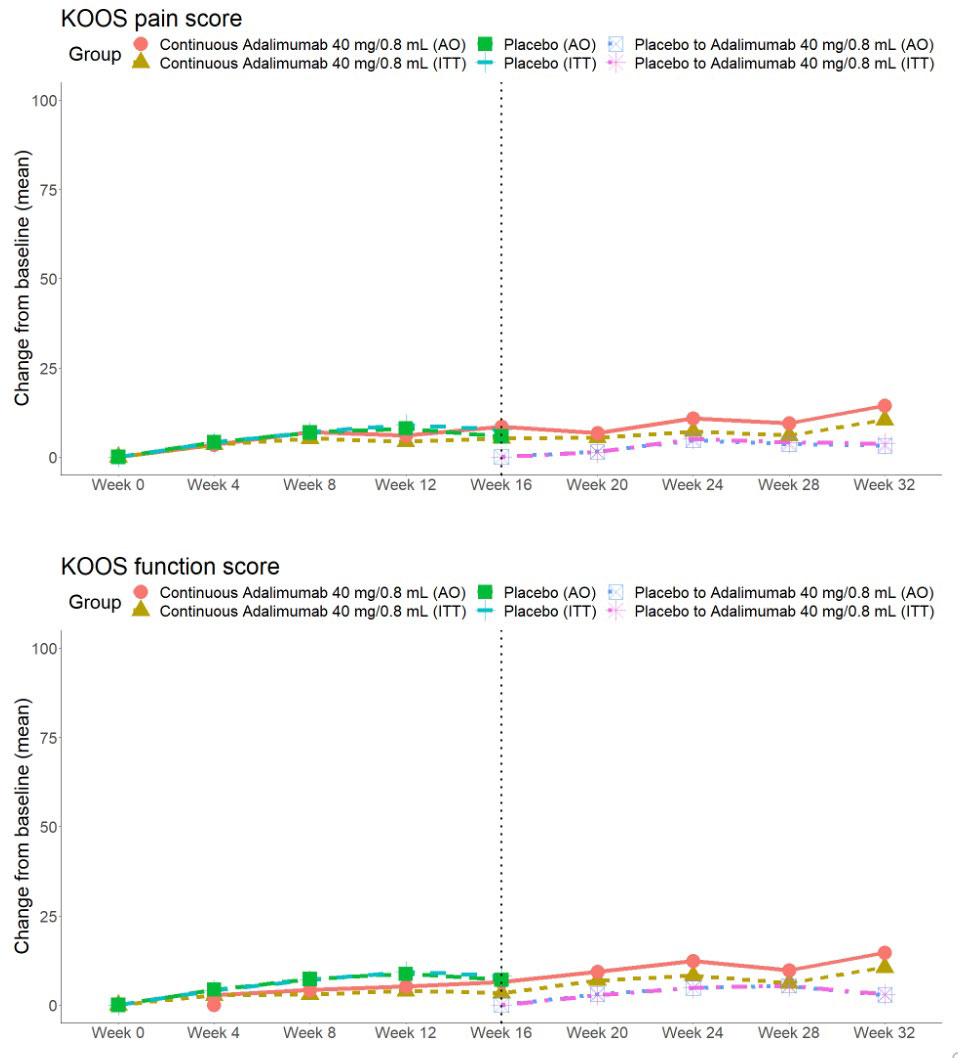
Results: A total of 58 patients were randomized (28 to PBO, 30 to ADA) and patients were well-matched for baseline characteristics (Table). The primary endpoint was not met: OMERACT-OARSI combined (ADA: 9 [30.0%] vs PBO: 7 [25%], p=0.90) (Figure 1). For KOOS pain, ≥20% improvement was noted in 11 (36.7%) ADA vs 7 (25.0%) PBO patients (p=0.50), and ≥50% improvement in 5 (16.7%) ADA vs 6 (21.4%) PBO patients (p=0.90). There were no significant treatment-group differences in baseline to 16-week change in continuous secondary endpoints (KOOS ADL-QoL-Symptoms-SRF, PGAD, IGAD, TJA, physical assessment of the target knee) or in lab markers (ESR, CRP) (Figure 2). OMERACT-OARSI response increased to 53.3% (ITT) at week 32 in the group randomized to ADA. There were 9 withdrawals (4 ADA, 5 PBO) to week 16 and 3 withdrawals between weeks 16-32 but no new safety signals were identified and there were no serious adverse events.
Conclusion: Although the treatment was safe, short-term treatment with anti-TNFα therapy does not appear to provide clinically meaningful improvements in OA symptoms though it remains possible that longer duration of treatment could be beneficial. Analyses of structural endpoints will be reported when results are available.
To cite this abstract in AMA style:
Maksymowych W, Bessette L, Lambert R, Carapellucci A, Appleton T. Osteoarthritis of the Knee, Inflammation, and the Effect of Adalimumab: A Randomized Placebo-Controlled Trial [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/osteoarthritis-of-the-knee-inflammation-and-the-effect-of-adalimumab-a-randomized-placebo-controlled-trial/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/osteoarthritis-of-the-knee-inflammation-and-the-effect-of-adalimumab-a-randomized-placebo-controlled-trial/