Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: The major obstacles to successful use of curcumin as an anti-inflammatory agent are its low solubility in water and rapid metabolism, which both translate to poor systemic bioavailability. To protect against metabolism and to enhance accessibility systemically, we have previously reported a novel formulation of nano-emulsified curcumin (NEC) that increases relative bioavailability by over 10-fold. In this study, we validated the bioactivity of NEC in vivo and characterized the associated mechanisms of immunosuppression.
Methods: Since curcumin has been shown to inhibit NFkB activation, we used BALB/C-Tg(NFkB-RE-luc)-Xen mice, which contain a firefly luciferase reporter gene under the control of kB responsive elements. Mice were treated with identical concentrations of curcumin in aqueous suspension (SC) or NEC by oral gavage and subsequently challenged with LPS. Acute systemic inflammation was measured and quantitated on the Xenogen in vivo imaging system (IVIS 200). Whole blood was collected for flow cytometry and serum was analyzed by ELISA. Acute peritonitis was induced in BALB/C mice by thioglycollate injection with and without NEC administration by oral gavage; peritoneal lavages were isolated for flow cytometry. Scratch assays to measure cell migration were performed on a human cells.
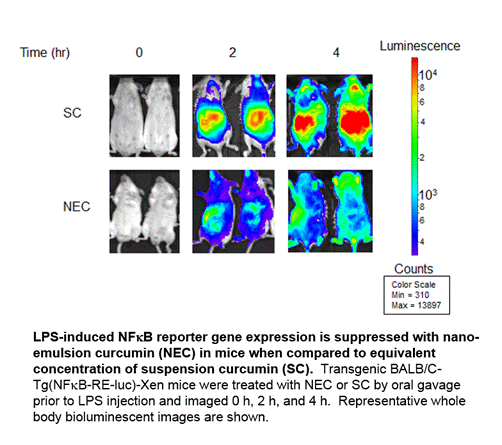
Results: Treatment with NEC significantly reduced LPS-induced inflammation relative to SC, as measured by IVIS detection of NFkB activity. Flow cytometry of peripheral blood indicated that circulating monocytes were reduced with administration of NEC and levels of LPS receptors TLR4 and RAGE were down-regulated on the surface of cells. Induction of monocyte chemoattractant protein (MCP)-1 secretion by LPS stimulation was significantly reduced with NEC administration. While macrophage recruitment was significantly reduced with NEC administration in thioglycollate-induced peritonitis, levels of T-cells and B-cells were not affected. Scratch assays to measure in vitro cell migration showed that curcumin significantly inhibited responses in both THP-1 cells and primary human macrophages.
Conclusion: In this study, we establish a novel system to screen and validate curcumin-derived drugs for longitudinal analysis in vivo. Furthermore, our results validate NEC as a candidate for future therapeutic studies and demonstrate that curcumin can suppress inflammation by selectively inhibiting macrophage migration via NFkB and MCP-1 inhibition.
Disclosure:
N. A. Young,
None;
M. Bruss,
None;
M. Gardner,
None;
W. Willis,
None;
X. Mo,
None;
G. Valiente,
None;
Y. Cao,
None;
Z. Liu,
None;
L. C. Wu,
None;
W. N. Jarjour,
None.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/oral-administration-of-nano-emulsion-curcumin-in-mice-suppresses-inflammatory-induced-nfkb-signaling-and-macrophage-migration/