Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Patients with rheumatoid arthritis are at increased risk for Herpes Zoster (HZ) infection, which is associated with increased hospitalizations and healthcare resource utilization. Despite recommendations by the American College of Rheumatology (ACR) and Advisory Committee on Immunization Practices (ACIP), vaccination against HZ for patients with rheumatoid arthritis between the ages of 18 to 49 years is low. Through a quality improvement approach, we aimed to increase the vaccination rate among RA patients between ages 18 and 49 years from 8% to 11% at a single academic center.
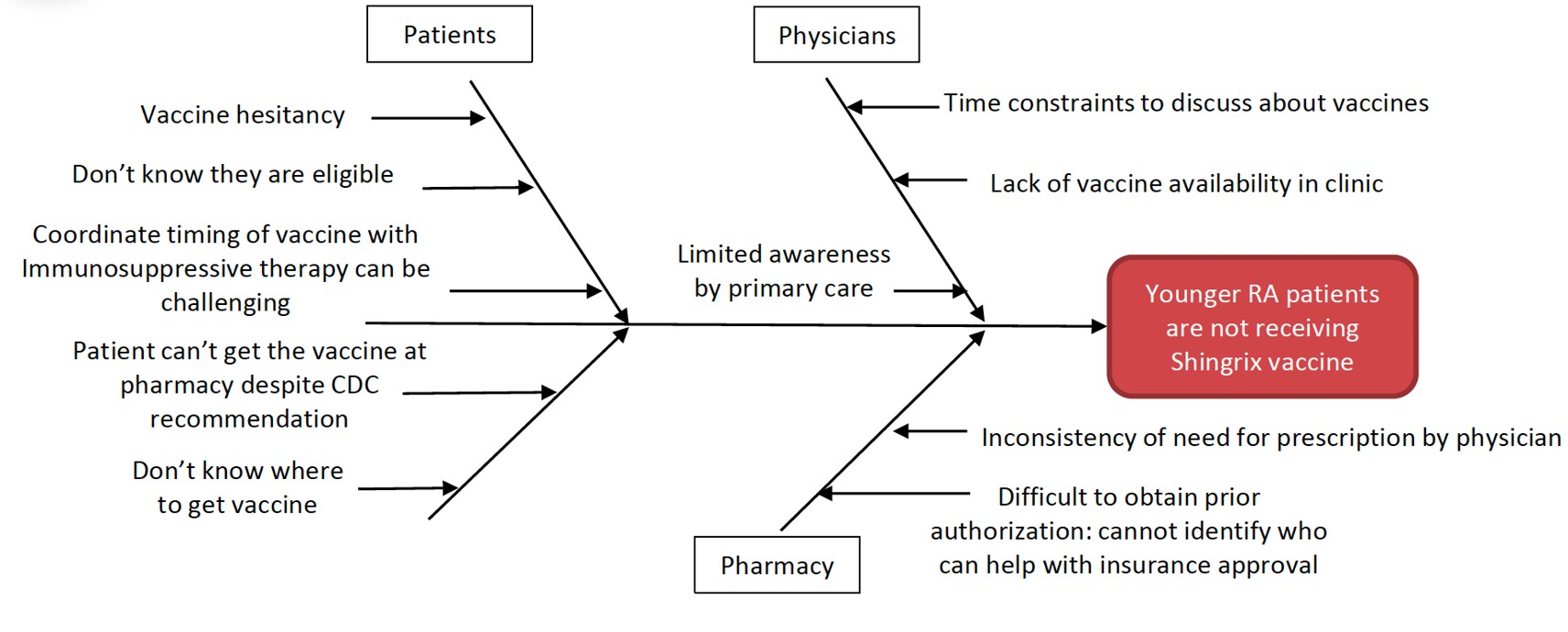
Methods: The baseline group was RA patients < 50 years of age seen at the Stanford Rheumatology Clinic between July to December 2023. Through root cause analysis (RCA), patient, physician, and pharmacy factors were identified as barriers to HZ vaccination. Initially, a scripted pre-visit phone call by clinic staff to eligible patients was performed to improve vaccine awareness among patients. Then, an effort to raise HZ vaccine awareness for patients < 50 years old was implemented through a monthly newsletter sent to over 200 primary care physicians at Stanford. With these efforts, the vaccination rate improvement was minimal. Subsequently, a targeted effort to improve vaccine access was implemented by collaborating with the Stanford Hospital Pharmacy, which is located next to the Rheumatology Clinic. Our clinic team was able to help obtain prior authorization for those whose insurance was required. Finally, a MyHealth message was sent to all eligible patients from the baseline group to alert them about vaccine availability at Stanford. At the end of the intervention period, the proportion of eligible patients who received a dose of the HZ vaccine was measured.
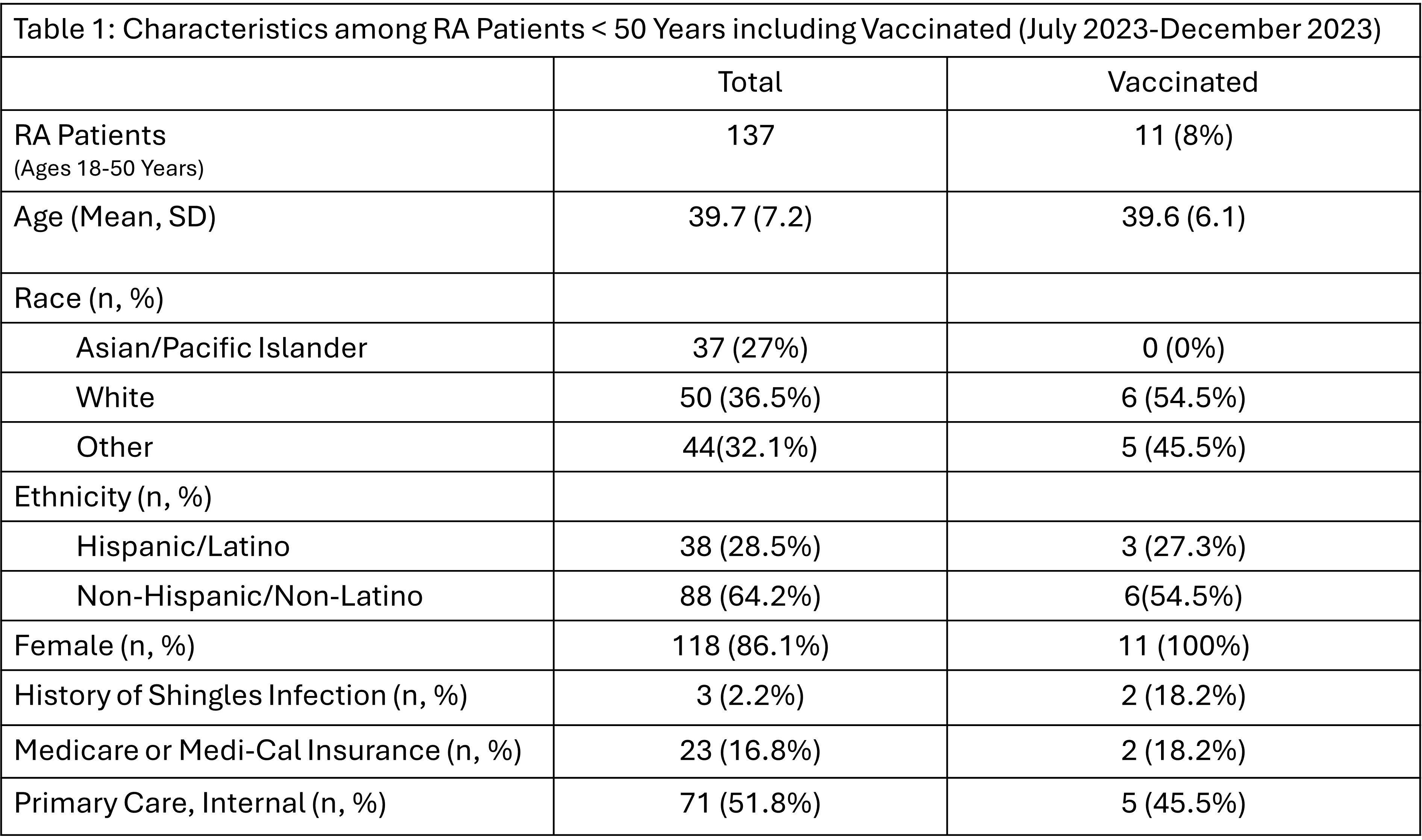
Results: There were 137 eligible RA patients < 50 years old who were seen at Stanford Rheumatology Clinic from July 2023 to December 2023. Table 1 highlights the characteristics of the baseline group, which only 11 (8%) have received the HZ vaccine. By the end of the 5-month intervention period, the proportion of eligible patients in the baseline group who received the vaccine increased from 8% to 11.7%. Additionally, 16.5% of eligible patients (n=20) expressed interest in receiving the vaccine, with subsequent clinic-initiated orders placed for these patients.
Conclusion: Vaccination against herpes zoster is sub-optimal among RA patients < 50 years. Targeting interventions at the patient, physician, pharmacy, insurance prior authorization, and institutional levels are critical to increase this population’s awareness and uptake of the HZ vaccine. It is promising that improving vaccine access for eligible patients appears to be the most successful. Additional steps are necessary to streamline the vaccine prior authorization process and further optimize the accessibility of the HZ vaccine at the institutional level.
To cite this abstract in AMA style:
Sood A, Jahangiri P, Bill C, Lin J. Optimizing Herpes Zoster Vaccination in Rheumatoid Arthritis Patients Ages 18-49 Years: A Quality Improvement Approach [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/optimizing-herpes-zoster-vaccination-in-rheumatoid-arthritis-patients-ages-18-49-years-a-quality-improvement-approach/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/optimizing-herpes-zoster-vaccination-in-rheumatoid-arthritis-patients-ages-18-49-years-a-quality-improvement-approach/