Session Information
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: CLIPPER2 was an 8-year, open-label extension of the phase 3b, multicenter, 2-year CLIPPER study of the safety and efficacy of etanercept (ETN) in the treatment of patients (pts) with juvenile idiopathic arthritis (JIA) categorized as extended oligoarticular arthritis (eoJIA), enthesitis-related arthritis (ERA), or psoriatic arthritis (PsA). The objective of this analysis was to describe the safety of ETN in this population after 10-years of follow up.
Methods: Pts (n=127) with eoJIA (2-17 years), ERA, or PsA (each 12-17 years) who received ≥1 ETN dose (0.8 mg/kg once weekly [max, 50 mg]) in CLIPPER were eligible to enter CLIPPER2. The primary outcome measure was the occurrence of malignancy. Long-term safety was assessed as the total incidence of events from CLIPPER baseline (BL) to month (m) 120, frequency of events per 100 patient-years (EP100PY), and frequency of events in each study year.
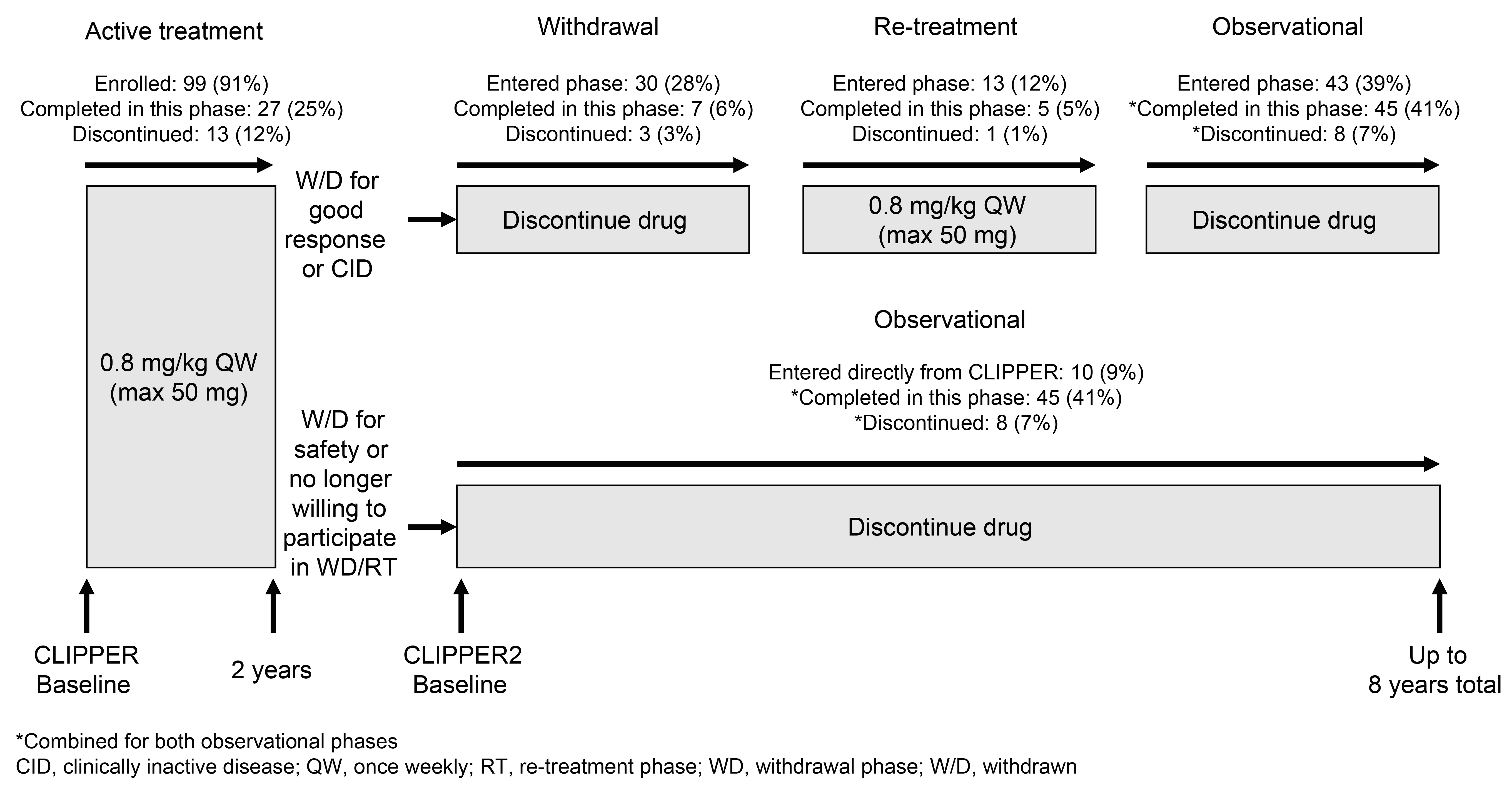
Results: A total of 109/127 (86%) pts entered CLIPPER2; 99 (91%) continued in the active treatment period. At m120, 84/109 (77%) pts had completed the study; 27 (25%) while actively taking ETN, 7 (6%) had withdrawn from treatment due to low/inactive disease; 5 (5%) had re-started ETN following an earlier withdrawal from treatment; and 45 (41%) had stopped ETN (but remained under observation) (Figure 1); 25/109 (23%) pts permanently discontinued from the CLIPPER2 study.
In CLIPPER/CLIPPER2, 1 case of malignancy (Hodgkin’s disease) was reported (1 pt with eoJIA in year 3). There was 1 case of uveitis (1 pt with eoJIA in year 8) and 3 of Crohn’s disease (2 pts with ERA, year 1/year 6; 1 pt with eoJIA, year 5). There were 2 cases of opportunistic infections (both herpes zoster), and no deaths. Overall, there were 559 (81.82 EP100PY) treatment-emergent adverse events (TEAEs) excluding infections and injection-site reactions (ISRs). The overall rate of TE serious infections was low (N=14; 2.05 EP100PY) (Table), with the most common TE serious infection being gastroenteritis (N=2; 0.29 EP100PY). The most frequently reported TEAEs (N [EP100PY]) were headache (28 [4.10]), arthralgia (24 [3.51]), pyrexia (21 [3.07]), diarrhea (14 [2.05]), and leukopenia (12 [1.76]). Overall, 39 patients reported serious TEAEs (excluding infections/ISRs).
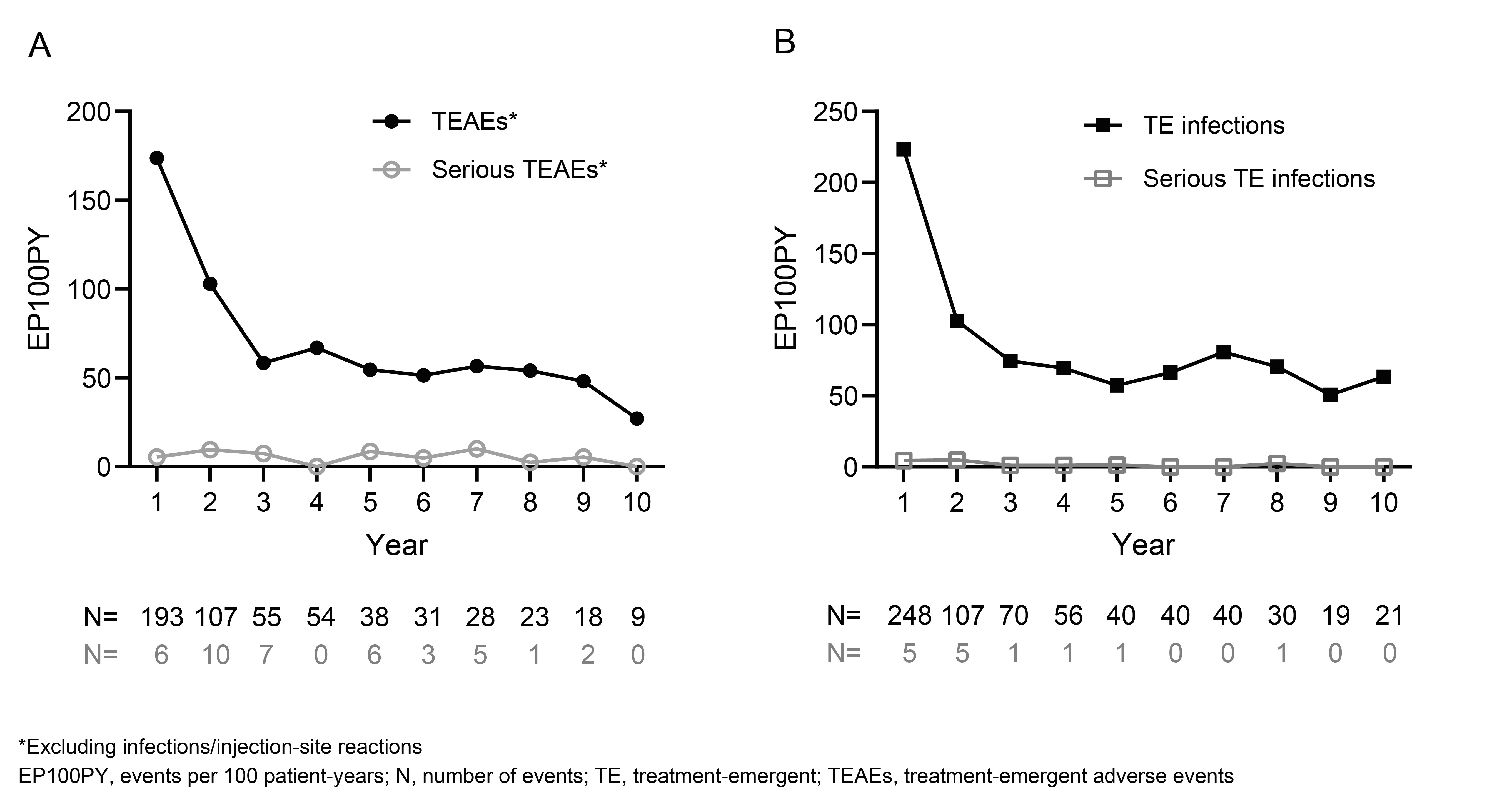
The number and frequency (N [EP100PY]) of TEAEs (excluding infections/ISRs) decreased over the 10-year study period from 193 [173.81] in year 1 to 9 [27.15] in year 10. The number and frequency of TE infections and serious TE infections also decreased over the 10-year study period. There was no clear trend of a decrease over time for the incidence of serious TEAEs (Figure 2).
Conclusion: ETN treatment to m120 was well tolerated in this patient population and consistent with the known safety profile. Frequency of TEAEs and TE infections decreased over time. Over 10 years there was I reported event of malignancy and the overall rate of TE serious infections was low.
Trial Registration: NCT00962741/NCT01421069
To cite this abstract in AMA style:
Vojinović J, Dehoorne J, Panaviene V, Susic G, Horneff G, Stanevicha V, Kobusinska K, Zuber Z, Dobrzyniecka B, Akikusa J, Avcin T, Martini A, Borlenghi C, Arthur E, Tatulych S, Zang C, Vlahos B, Ruperto N. Open-label, Long-term (10-year) Study of the Safety of Etanercept in Children with Extended Oligoarticular Arthritis, Enthesitis-related Arthritis, or Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/open-label-long-term-10-year-study-of-the-safety-of-etanercept-in-children-with-extended-oligoarticular-arthritis-enthesitis-related-arthritis-or-psoriatic-arthritis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/open-label-long-term-10-year-study-of-the-safety-of-etanercept-in-children-with-extended-oligoarticular-arthritis-enthesitis-related-arthritis-or-psoriatic-arthritis/