Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Olokizumab (OKZ), an interleukin-6 inhibitor approved in several countries for managing rheumatoid arthritis (RA), was evaluated in previous phase III RCTs, demonstrating changes in patient-reported outcomes (PROs) for MTX-IR and TNFi-IR patients with active disease [1-3]. This study extends those findings to assess the long-term sustainability of OKZ’s effects on PROs up to 106 weeks (ClinicalTrials.gov number, NCT03120949).
Methods: We included all randomized for OKZ or PBO in phase III clinical program patients (with active rheumatoid arthritis and fulfilled the ACR/EULAR 2010 revised classification criteria). We analyzed data using the Intent-To-Treat population, utilizing a mixed model for repeated measures to assess changes from baseline. Results are expressed as least squares mean (LSM) and standard error. The proportion of patients reporting improvement from baseline at week 24 ≥ the minimal clinically important difference (MCID) in HAQ-DI scores was determined using thresholds of ≥0.22 and ≥0.3 points. Post hoc responder analyses were conducted to estimate the percentages of patients who reported improvement from baseline ≥ MCID of 10 mm for PtGA and pain VAS scores; 2.5 points for SF-36 PCS and MCS scores, 5 points for individual domains; and 4 points for the FACIT-F. Discontinuations or use of rescue medication classified subjects as non-responders.
Results: Overall 1982 patients were included. MTX-IR and TNFi-IR populations were comparable at baseline with a mean age of 53 years, mostly women (77-90%), 56-61% using glocucorticoids, and DAS28-CRP approximately 5.87-5.99 (RA duration, CDAI, intolerance to ≥15 mg/week MTX dose and HAQ-DI were numerically higher in TNFi-IR patients). At week 12, OKZ biweekly (q2w), and every four weeks (q4w), compared with placebo showed significantly greater LSM improvements in all PROs for MTX-IR patients (Table 1). For TNFi-IR patients, improvements were not as large as in MTX-IR population (Table 2). PRO improvements were sustained through week 106. Post hoc analyses demonstrated a higher proportion of patients receiving OKZ attained improvements ≥ MCID vs. placebo (p< 0.05) at week 12 in all (except EQ-5D) reported PROs for both MTX-IR and TNFi-IR. Numbers needed to treat (NNT) ranged from 4.6 to 11.6 with OKZ q2w vs 10.6 to 13.5 with OKZ q4w (MTX-IR), and from 7.6 to 27.8 and 7.5 to 34.5 (TNFi-IR), accordingly. SF-36 domain changes from baseline to week 106 presented (Figure 1).References 1. Nasonov E, et al. Ann Rheum Dis. 2022;81(4):469-479.2. Smolen JS, et al. N Engl J Med. 2022;387(8):715-726. 3. Feist E, et al. Ann Rheum Dis. 2022 Dec;81(12):1661-1668.
Conclusion: Treatment with OKZ over 106 weeks resulted in statistically significant and clinically meaningful improvements in PROs compared to baseline in both MTX-IR and TNFi-IR RA patients. The improvements in PRO’s were observed as early as week 12, were sustained and improved further to week 106. There were no discernible differences between the two regimens of OKZ regarding patient reported outcomes.
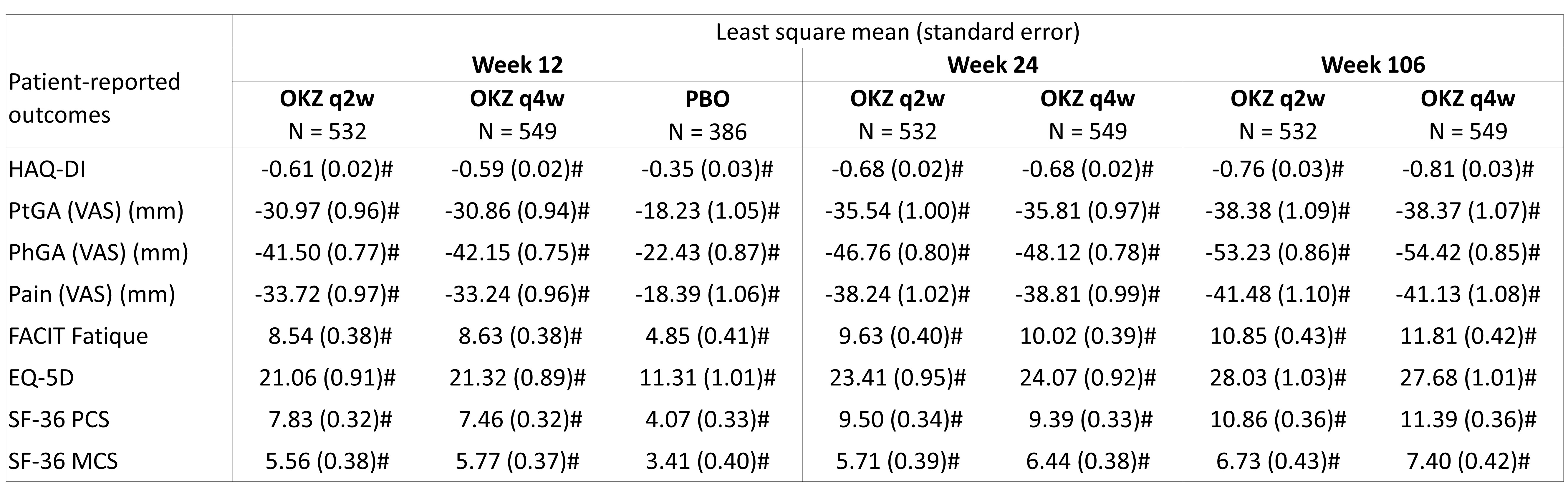 Table 1. Change from baseline in patient-reported outcome scores at weeks 12, 24 and 106 (as observed). MTX-IR
Table 1. Change from baseline in patient-reported outcome scores at weeks 12, 24 and 106 (as observed). MTX-IR
#p < 0.050 compared to the baseline value within the group.
All p values are nominal.
FACIT-F, Functional Assessment of Chronic Illness Therapy–Fatigue scale; HAQ-DI, Health Assessment Questionnaire Disability Index; MCS, mental component summary; PCS, physical component summary; PtGA, patient global assessment of disease activity; q2w, every 2 weeks; q4w, every 4 weeks; SF-36, Short Form 36 Health Survey; VAS, visual analogue scale.
.jpg) Table 2 Change from baseline in patient-reported outcome scores at weeks 12, 24 and 106 (as observed). TNFa-IR
Table 2 Change from baseline in patient-reported outcome scores at weeks 12, 24 and 106 (as observed). TNFa-IR
#p < 0.050 compared to the baseline value within the group.
All p values are nominal.
FACIT-F, Functional Assessment of Chronic Illness Therapy–Fatigue scale; HAQ-DI, Health Assessment Questionnaire Disability Index; MCS, mental component summary; PCS, physical component summary; PtGA, patient global assessment of disease activity; q2w, every 2 weeks; q4w, every 4 weeks; SF-36, Short Form 36 Health Survey; VAS, visual analogue scale.
.jpg) Figure 1. SF-36 domain changes from baseline to week 106
Figure 1. SF-36 domain changes from baseline to week 106
Abbreviations: AGNorms, age- and gender-matched normative values; BP, bodily pain; GH, general health; MTX-IR, methotrexate irresponsive; MH, mental health; OKZ, olokizumab; PF, physical functioning; PBO, placebo; RE, role emotional; RP, role physical; SF-36, the Short Form health survey is a 36-item, patient-reported survey; SF, social functioning; TNFi-IR, tumor necrosis factor inhibitors irresponsive; VT, vitality
To cite this abstract in AMA style:
Fleischmann R, feist E, Smolen J. Olokizumab Improves Patient-Reported Outcomes in Rheumatoid Arthritis MTX-IR and TNF-IR Patients up to 106 Weeks (Results from Clinical Phase III Program) [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/olokizumab-improves-patient-reported-outcomes-in-rheumatoid-arthritis-mtx-ir-and-tnf-ir-patients-up-to-106-weeks-results-from-clinical-phase-iii-program/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/olokizumab-improves-patient-reported-outcomes-in-rheumatoid-arthritis-mtx-ir-and-tnf-ir-patients-up-to-106-weeks-results-from-clinical-phase-iii-program/
