Session Information
Date: Saturday, November 12, 2022
Title: RA – Treatment Poster I
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Olokizumab (OKZ) is an interleukin-6-inhibitor for treatment of rheumatoid arthritis (RA). In these analyses we present patient reported outcomes (PROs) reported by TNF-IR patients with moderate to severely active RA receiving OKZ or placebo in a phase III randomized controlled trial (RCT) CREDO3 (ClinicalTrials.gov number, NCT02760433).1
To assess the effect of OKZ treatment compared with placebo in patient global assessment of disease activity (PtGA), pain, physical function (HAQ-DI), fatigue (FACIT-F) and health related quality of life (SF-36 physical (PCS) and mental (MCS) component summary and domain scores) at 12 weeks.
Methods: 368 patients were randomized 2:2:1 to receive subcutaneously administered OKZ 64 mg once every 2 weeks (q2w), OKZ 64 mg q4w, or placebo, plus MTX. PROs were assessed at baseline, weeks 12 (primary endpoint) and 24. At week 16, all patients receiving placebo were switched to either OKZ dose. Between groups differences in least-squares mean (LSM) changes from baseline were analyzed, p < 0.05 considered significant; nominal p-values for PROs not in the hierarchy.
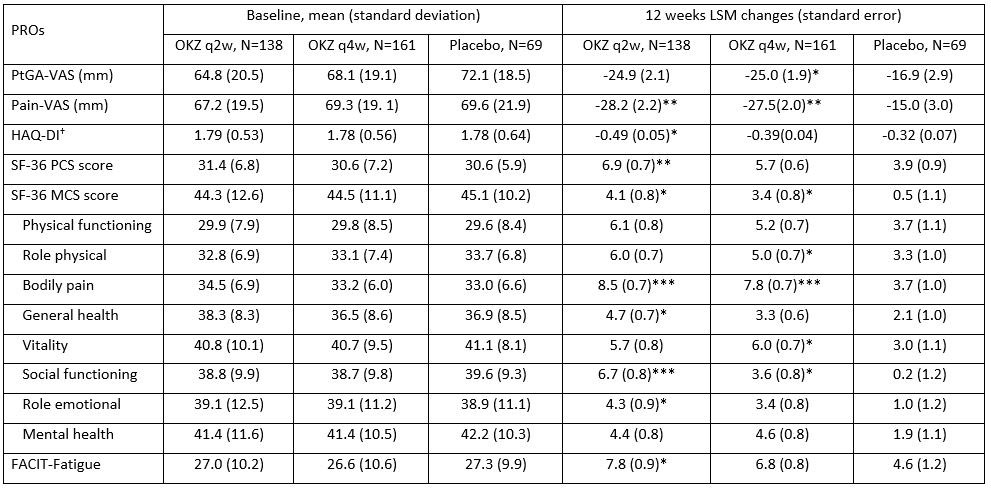
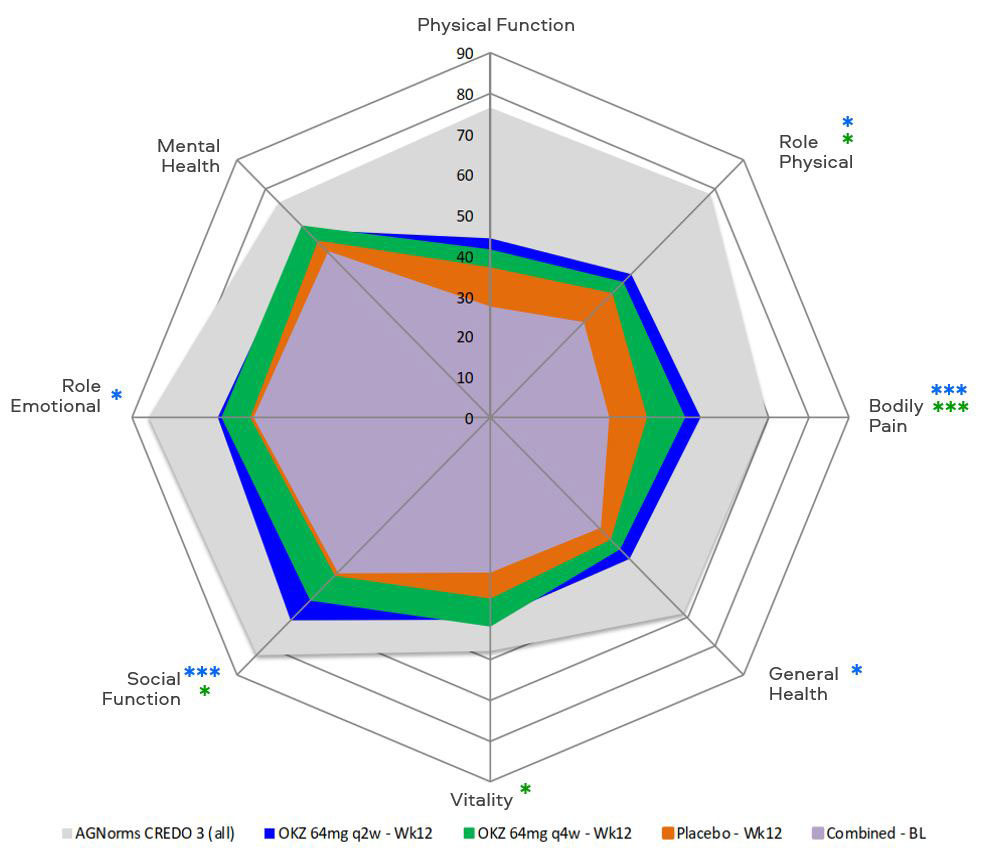
Results: Baseline demographics and disease characteristics were comparable between groups. At week 12, treatment with OKZ q2w compared with placebo resulted in significantly greater LSM changes from baseline in Pain, HAQ-DI, FACIT-F, SF-36 PCS, MCS and 4 domains; with OKZ q4w in PtGA, Pain, SF-36 MCS and 4 domains (Table, Figure). Improvements reported at week 12 in PROs continued or increased with both doses of OKZ until week 24. Post hoc analyses demonstrated that a higher proportion of patients receiving OKZ reported improvements ≥minimum clinically important differences vs placebo (p< 0.05) in FACIT-F, SF-36 PCS and MCS scores, indicating that these changes translated into clinically meaningful benefits on an individual patient basis. Numbers needed to treat to gain these benefits in fatigue and physical function ranged from 9.2 – 15.4 with OKZ q2w vs 10.5 – 13.3 with OKZ q4w, respectively.
Conclusion: Treatment with OKZ over 12 weeks resulted in statistically significant improvements in PROs vs placebo reported by TNF-IR RA patients. Benefits were more frequently reported by patients receiving OKZ q2w than q4w in this phase 3 RCT of limited size in treatment experienced patients.
Footnotes: NRI for Missing data.
†, secondary endpoint; *p≤0.05, **p<0.01, ***p<0.001 vs placebo.
To cite this abstract in AMA style:
Strand V, Nasonov E, Lisitsyna T, Lila A, Kuzkina S, Samsonov M, Feist E. Olokizumab Improved Patient Reported Outcomes in TNF Incomplete Responder (TNF-IR) Rheumatoid Arthritis Patients: Results from the Phase III Randomized Controlled Trial [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/olokizumab-improved-patient-reported-outcomes-in-tnf-incomplete-responder-tnf-ir-rheumatoid-arthritis-patients-results-from-the-phase-iii-randomized-controlled-trial/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/olokizumab-improved-patient-reported-outcomes-in-tnf-incomplete-responder-tnf-ir-rheumatoid-arthritis-patients-results-from-the-phase-iii-randomized-controlled-trial/