Session Information
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Subcutaneous (SC) tanezumab, a monoclonal antibody that inhibits nerve growth factor, was investigated for the relief of signs and symptoms of moderate-severe OA in patients (pts) for whom use of other analgesics was ineffective or not appropriate in a randomized NSAID-controlled long-term global safety study (NCT02528188). The primary safety (joint safety over 80 weeks [Wk]) and coprimary efficacy endpoints (pain, function, and Pt Global Assessment of OA [PGA-OA] at Wk 16) were recently disclosed.1,2 The current analysis examines the time-course and longer term maintenance of treatment effect of tanezumab versus NSAID on pain and physical function through Wk 56.
Methods: Eligible pts had hip or knee OA based on ACR criteria with x-ray confirmation; baseline (BL) WOMAC Pain and Physical Function subscale scores of ≥5; BL PGA-OA of “fair”, “poor”, or “very poor”; history of inadequate pain relief with acetaminophen; inadequate pain relief with/intolerance tramadol or opioids, or unwilling to take opioids. Pts were on a qualifying, stable dose of NSAID before study entry. Pts received SC tanezumab (2.5 mg or 5 mg every 8 Wks) or oral NSAID (BID) over the 56-Wk treatment period. At Wk 16, pts who did not have the required efficacy response (≥15% reduction from BL at Wk 2, 4, or 8, and ≥30% reduction from BL in WOMAC Pain at Wk 16) were discontinued from study treatment and entered the safety follow-up period. Pts were permitted to use rescue medication (≤3000 mg/day acetaminophen) up to 3 days/Wk up to Wk 16 and daily thereafter. Treatment effect through Wk 56 versus NSAID was evaluated by measuring change from BL in average index joint pain (electronic pain diary: daily from Day 1 to Wk 16, then weekly to end of study) and WOMAC Pain and Physical Function, and PGA-OA scores (Wk 2 through Wk 56 at clinic visits). Unadjusted p-values are presented.
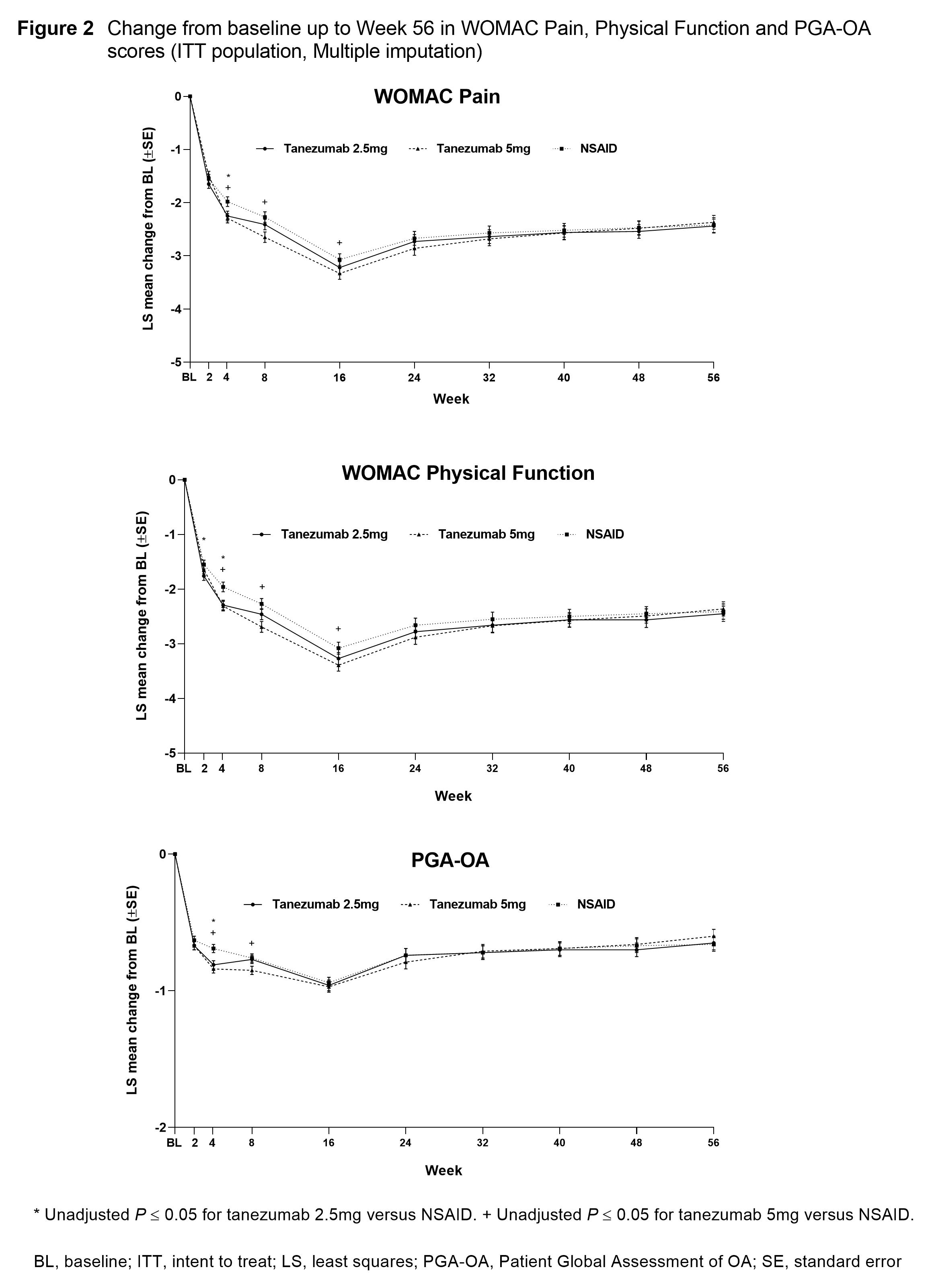
Results: In total, 3,021 pts were randomized and 2,996 included in the efficacy analyses. A total of 1,312 pts completed the treatment period (42–45% across treatment groups). BL pt characteristics were similar across treatment groups: mean age 60.3–61.2 years; duration of index joint OA 7.9–8.1 years; index joint was the knee 84.9–85.5%; female 63.6–66.5%. Reductions from BL in average index joint pain scores were largely similar across treatment groups from BL to Wk 4 (Figure 1). From Wk 4 to Wk 20 reductions were significantly greater (P≤0.05) in both tanezumab groups versus NSAID. Changes from BL in WOMAC Pain, Physical Function and PGA-OA scores were largely similar across treatment groups but there were scattered significant improvements for pts receiving tanezumab versus NSAID at Wks 4, 8 and 16 (Figure 2). Changes in WOMAC Pain, Physical Function and PGA-OA scores across treatment groups were generally maintained up to Wk 56.
Conclusion: Pts with moderate-severe hip or knee OA treated with tanezumab or NSAID experienced an early improvement in pain and function that was maintained long-term.
Disclosures: Sponsored by Pfizer and Eli Lilly & Company.
References:
- Hochberg M, et al. ACR/ARHP 2019 Annual Scientific Meeting. 2019;71(S10):4888.
- Hochberg M, et al. ACR/ARHP 2019 Annual Scientific Meeting. 2019;71(S10):2243.
To cite this abstract in AMA style:
Neogi T, Hunter D, Churchill M, Shirinsky I, Omata M, White A, Guermazi A, Fountaine R, Pixton G, Viktrup L, Brown M, West C, Verburg K. Observed Efficacy with Subcutaneous Tanezumab Is Early and Maintained in Patients with Osteoarthritis: Results from a 56-Week Randomized NSAID-Controlled Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/observed-efficacy-with-subcutaneous-tanezumab-is-early-and-maintained-in-patients-with-osteoarthritis-results-from-a-56-week-randomized-nsaid-controlled-study/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/observed-efficacy-with-subcutaneous-tanezumab-is-early-and-maintained-in-patients-with-osteoarthritis-results-from-a-56-week-randomized-nsaid-controlled-study/