Session Information
Date: Saturday, November 16, 2024
Title: SLE – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
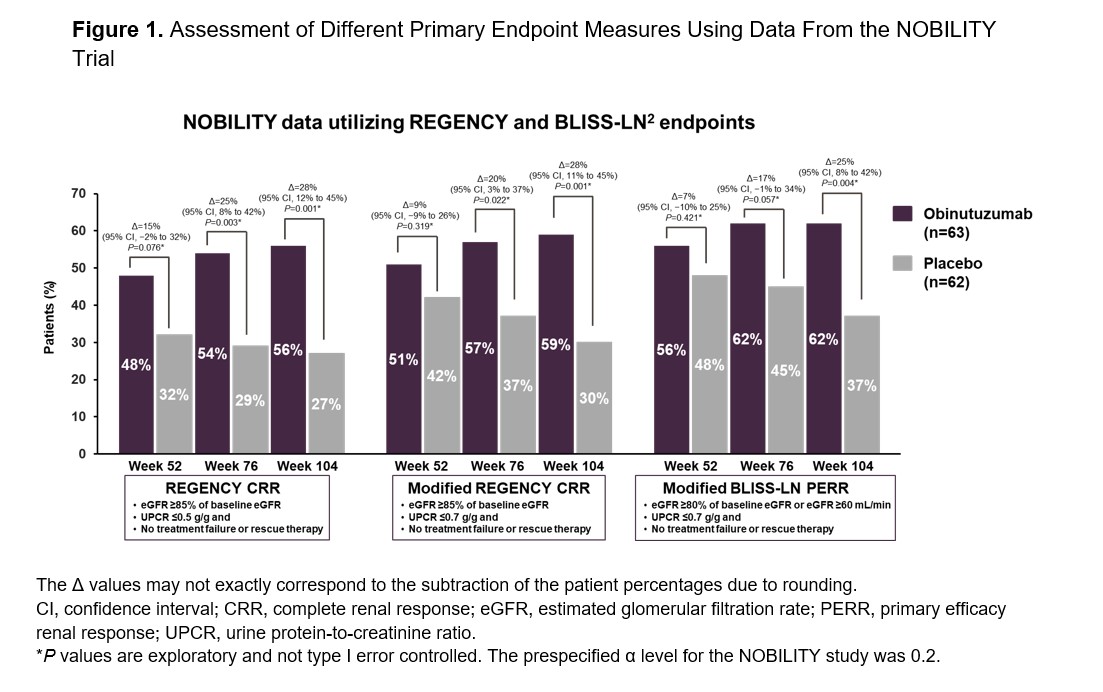
Background/Purpose: Lupus nephritis (LN) is the most common severe organ-threatening manifestation of systemic lupus erythematosus (SLE). The randomized, placebo-controlled, Phase II NOBILITY trial (NCT02550652) demonstrated superiority of obinutuzumab over placebo for achievement of complete and overall renal responses at Week 52, when added to standard-of-care mycophenolate mofetil and corticosteroids in patients with proliferative LN.1 Recent and ongoing registrational clinical trials in LN use composite primary endpoints driven primarily by reduction in proteinuria. Improvement of proteinuria to < 0.7-0.8 g/day after new-onset LN or LN flare positively influences long-term kidney health. This post hoc analysis assessed the achievement of complete renal response (CRR) in participants from the NOBILITY trial using primary endpoint definitions from the ongoing REGENCY as well as the BLISS-LN2 trials and evaluated the effect of baseline proteinuria on REGENCY CRR attainment.
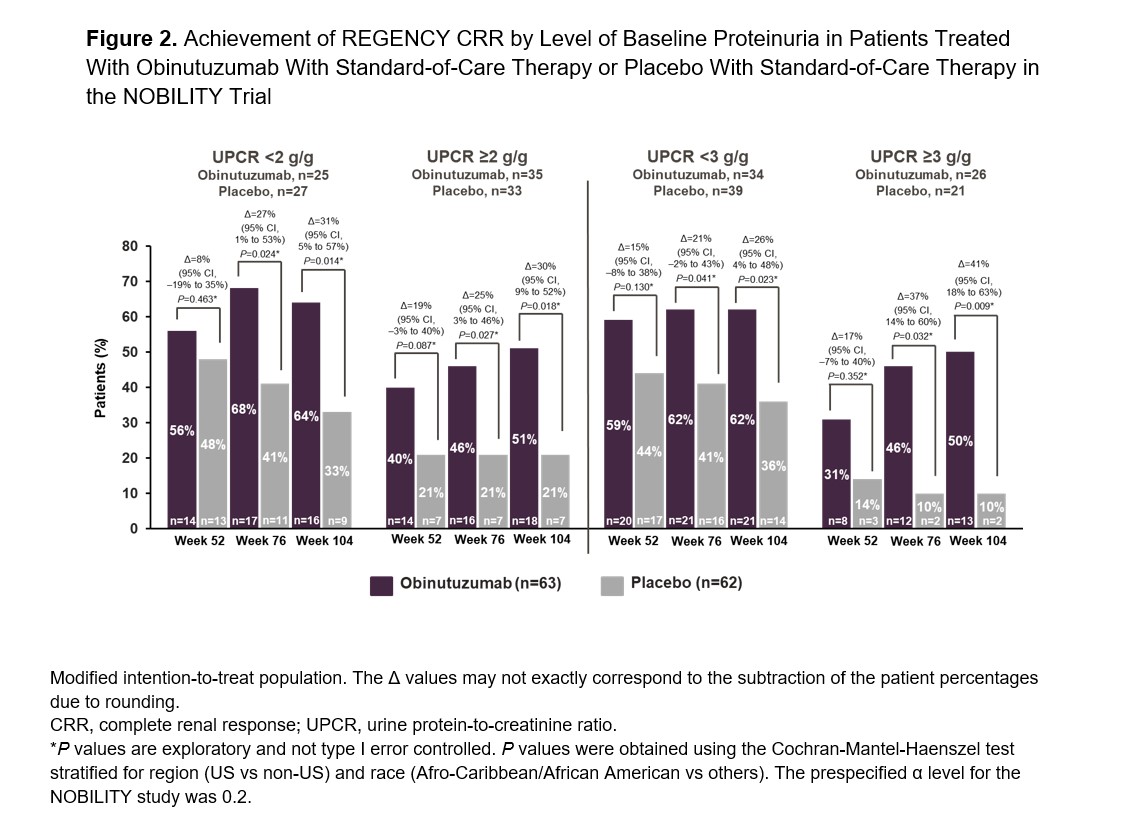
Methods: Primary and modified primary endpoints from the Phase III REGENCY trial (NCT04221477) and a modified primary endpoint from the Phase III BLISS-LN trial2 (NCT01639339) were used for this analysis at Weeks 52, 76 and 104. Endpoint measures were defined as follows: REGENCY CRR (estimated glomerular filtration rate [eGFR] ≥85% of baseline, urine protein-to-creatinine ratio [UPCR] ≤0.5 g/g and no treatment failure or rescue therapy), modified REGENCY CRR (eGFR ≥85% of baseline eGFR, UPCR ≤0.7 g/g and no treatment failure or rescue therapy) and modified BLISS-LN primary efficacy renal response (PERR; eGFR ≥80% of baseline eGFR or eGFR ≥60 mL/min, UPCR ≤0.7 g/g and no treatment failure or rescue therapy). Subgroup efficacy analyses were performed by baseline UPCR < 2 vs ≥2 g/g and < 3 vs ≥3 g/g. Cochran-Mantel-Haenszel test stratified for region (US vs non-US) and race (Afro-Caribbean/African American vs others) was used to compare the CRRs between the treatment arms. All patients met the American College of Rheumatology classification criteria for SLE and had biopsy-proven proliferative LN.
Results: Obinutuzumab with standard-of-care therapy was superior to placebo with standard-of-care therapy when using the REGENCY CRR definition with the existing UPCR ≤0.5 g/g or with the modified UPCR cutoff of ≤0.7 g/g at Weeks 76 and 104 as well as the BLISS-LN PERR definition at Week 104 (Figure 1). In addition, the efficacy of obinutuzumab administration with standard-of-care therapy compared to placebo with standard-of-care therapy was observed irrespective of baseline proteinuria levels (Figure 2).
Conclusion: The favorable effects of obinutuzumab, in combination with standard therapy, were replicated with the REGENCY and BLISS-LN primary endpoint definitions. Beneficial effects of obinutuzumab were also demonstrated regardless of baseline levels of proteinuria. Obinutuzumab is currently undergoing further evaluation in patients with active proliferative LN in the global, registrational, Phase III REGENCY trial (NCT04221477).
References
- Furie RA, et al. Ann Rheum Dis. 2022;81(1):100-107.
- Furie RA, et al. N Engl J Med. 2020;383(12):1117-1128.
To cite this abstract in AMA style:
Furie R, Terres J, Martins E, Hassan I, Schindler T, Garg J, Pendergraft III W, Malvar A, Rovin B. Obinutuzumab Benefits Patients with Active Lupus Nephritis Irrespective of Baseline Proteinuria Severity: A Post Hoc Analysis of a Phase II Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/obinutuzumab-benefits-patients-with-active-lupus-nephritis-irrespective-of-baseline-proteinuria-severity-a-post-hoc-analysis-of-a-phase-ii-trial/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/obinutuzumab-benefits-patients-with-active-lupus-nephritis-irrespective-of-baseline-proteinuria-severity-a-post-hoc-analysis-of-a-phase-ii-trial/