Session Information
Date: Monday, November 13, 2023
Title: (1052–1081) Immunological Complications of Medical Therapy Poster
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Immune checkpoint inhibitors (ICIs) are used to treat multiple cancers with increasing frequency and have led to improved survival. However, there is limited data on the use of ICIs in patients with pre-existing autoimmune disease, specifically rheumatoid arthritis (RA), as these patients were excluded from most clinical trials. There is concern that RA patients may experience higher mortality with ICI treatment. The objective of this investigation was to compare all-cause and cause-specific mortality following ICI treatment in patients with and without pre-existing RA in the Veterans Health Administration (VHA).
Methods: This analysis employed data from the VHA Corporate Data Warehouse (CDW) for demographic and pharmacy information, VA Central Cancer Registry for cancer diagnosis, Death Ascertainment File (DAF) for all-cause mortality rates, and National Death Index (NDI) for cause of death. We identified Veterans with RA in the VHA with two ICD codes at least 30 days apart, at least one ICD code from a rheumatologist, and treatment with a disease modifying anti-rheumatic drug. Each RA case was matched up to 10:1 based on year of birth, sex, and year of VHA enrollment to Veterans without RA. RA and non-RA patients receiving ICI therapy between 6/6/2011 and 2/14/2023 were identified.All-cause mortality up to 4/30/2023 was obtained from the DAF and cause specific mortality from NDI through 12/31/2019. Demographic, ICI treatment, and cancer type were compared using student t-test or Chi square. Survival from the time of ICI initiation was evaluated using Kaplan-Meier curves and log rank testing.
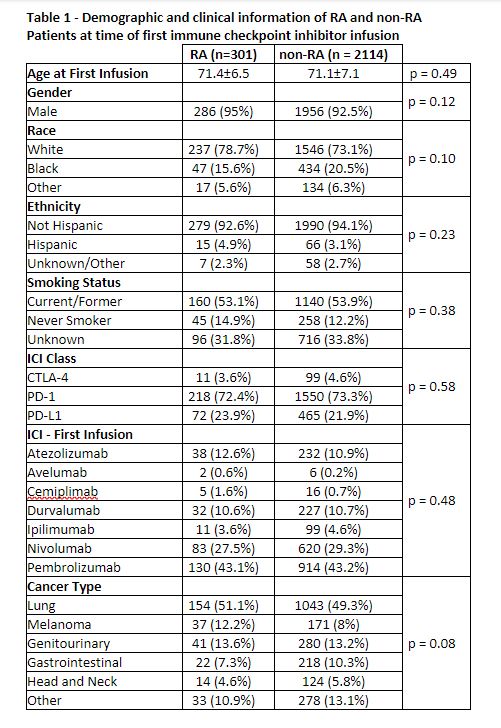
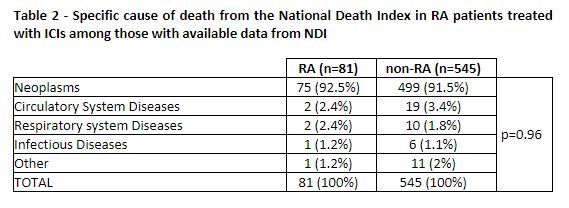
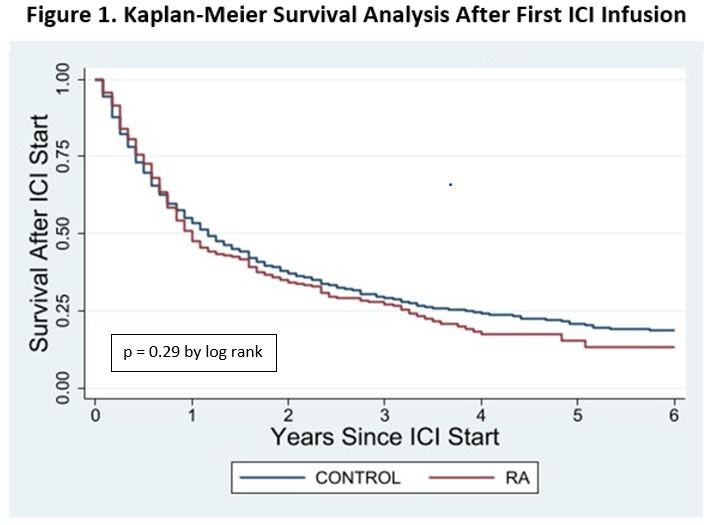
Results: The cohort of Veterans with RA included 73,677 patients matched to 727,627 controls. There were 301 (0.41%) among the RA patients and 2,114 (0.29%) among the non-RA controls treated with an ICI. There were no differences in demographics, smoking status, first ICI drug, or cancer diagnosis between these groups at the time of initial ICI infusion (Table 1). The Veterans were majority white, non-Hispanic, male, and current or former smokers. Lung cancer was the most common malignancy type and pembrolizumab was used most frequently as the first ICI in both groups. Both groups had high mortality with one-year survival 51.2% [45.2% – 56.7%] vs. 54.5% [45.3% – 56.7%] and two-year survival 35.0% [29.3% – 40.7%] vs. 37.5% [35.2% – 39.7%] in the RA and non-RA groups, respectively. There was no significant survival difference between the groups (p=0.29)(Figure 1). The NDI cause of death was similar in both groups (p=0.96) with the most common being neoplasm in 91.9% and 91.2% of patients, respectively (Table 2). Deaths due to infection were rare in both groups (1.2%).
Conclusion: Veterans with pre-existing RA who received ICIs for cancer did not experience excess mortality or differences in cause of death compared to Veterans without RA receiving ICI treatment. These preliminary data suggest that a diagnosis of RA alone should not serve as a contraindication to ICI therapy in the context of comorbid cancer.
To cite this abstract in AMA style:
O'Sullivan M, Cannon G, Sauer B, Rojas Jr J, Kunkel G, Walsh J, Roul P, England B, Mikuls T, Baker J, Braaten T. No Increase in Mortality in US Veterans with Rheumatoid Arthritis Treated with Immune Checkpoint Inhibitors (ICIs) [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/no-increase-in-mortality-in-us-veterans-with-rheumatoid-arthritis-treated-with-immune-checkpoint-inhibitors-icis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/no-increase-in-mortality-in-us-veterans-with-rheumatoid-arthritis-treated-with-immune-checkpoint-inhibitors-icis/