Session Information
Date: Sunday, November 13, 2022
Title: Systemic Sclerosis and Related Disorders – Clinical Poster II
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: The Scleroderma Clinical Trials Consortium Damage Index (SCTC-DI) was developed to quantify organ damage in patients with SSc. We assessed outcomes in the SENSCIS trial of nintedanib versus placebo in subgroups by organ damage assessed using a modified version of the SCTC-DI.
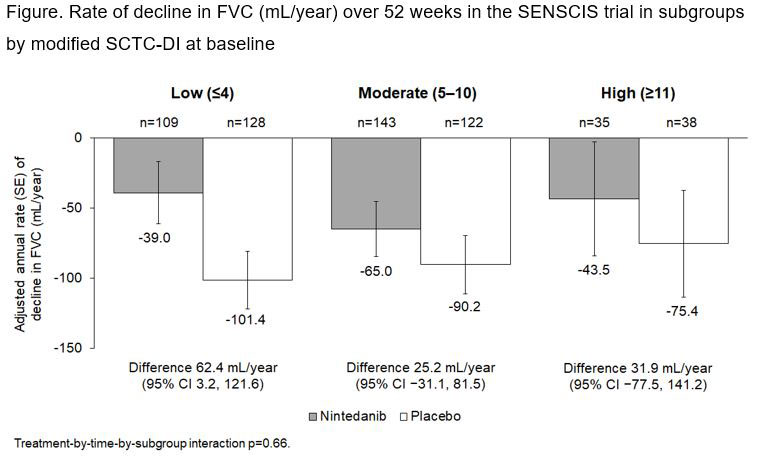
Methods: The SENSCIS trial enrolled patients with SSc with first non-Raynaud symptom in the prior ≤7 years, extent of fibrotic ILD on high-resolution computed tomography ≥10% and forced vital capacity (FVC) ≥40% predicted. Patients with clinically significant pulmonary hypertension were excluded. Patients were randomized to receive nintedanib or placebo until the last patient reached week 52 but for ≤100 weeks. The SCTC-DI captures damage attributable to SSc in 6 organ systems (musculoskeletal and skin, vascular, respiratory, gastrointestinal, cardiovascular, renal). For use in this analysis, a modified version of the SCTC-DI was developed in which items based on data that were not collected in the SENSCIS trial were excluded, items that were exclusion criteria were given a score of 0 (not fulfilled) for all patients, and the two items relating to joint contractures were merged. The modified version had a maximum total score of 43. We analyzed the rate of decline in FVC (mL/year) and adverse events over 52 weeks in subgroups by modified SCTC-DI score (≤4 ([low], 5–10 [moderate], ≥11 [high]) at baseline. An exploratory interaction p-value was calculated to assess potential heterogeneity in the effect of nintedanib versus placebo across these subgroups.
Results: Among 576 patients, 41.1%, 46.2% and 12.7% had low, moderate and high modified SCTC-DI scores, respectively, at baseline. The extent of fibrosis on HRCT and the degree of lung function impairment were greater in patients with high SCTC-DI scores than in patients with moderate scores, and greater in patients with moderate scores than low scores. In the placebo group, the rate of decline in FVC over 52 weeks was similar across the subgroups by modified SCTC-DI score (Figure). The effect of nintedanib on reducing the rate of FVC decline was numerically greater in patients with a low modified SCTC-DI score compared to patients with a moderate or high score, but the interaction p-value did not indicate heterogeneity (difference) in the effect of nintedanib across the subgroups (p=0.66). The adverse event profile of nintedanib was similar across the subgroups, but the proportion of patients with serious adverse events was greater in nintedanib-treated patients with a high modified SCTC-DI score than in those with a low or moderate score (Table).
Conclusion: Patients in the SENSCIS trial had varying severities of organ damage based on a modified version of the SCTC-DI. Nintedanib had a consistent effect on reducing the rate of FVC decline over 52 weeks across subgroups based on organ damage.
To cite this abstract in AMA style:
Pope J, Proudman S, Stevens W, Henes J, Simonovska R, Alves M, Allanore Y. Nintedanib in Patients with Systemic Sclerosis-Associated Interstitial Lung Disease (SSc-ILD) and Organ Damage: Data from the SENSCIS Trial [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/nintedanib-in-patients-with-systemic-sclerosis-associated-interstitial-lung-disease-ssc-ild-and-organ-damage-data-from-the-senscis-trial/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/nintedanib-in-patients-with-systemic-sclerosis-associated-interstitial-lung-disease-ssc-ild-and-organ-damage-data-from-the-senscis-trial/