Session Information
Date: Sunday, November 12, 2023
Title: Abstracts: B Cell Biology & Targets in Autoimmune & Inflammatory Disease
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Anti-citrullinated protein antibodies (ACPA) play a role in rheumatoid arthritis (RA) pathogenesis, and their presence is associated with disease severity. Consequently, detailed analysis of ACPApos B cells is required to disentangle the role of these cells in RA. Multiple intracellular signaling pathways are involved in functional B cell responses. NF-κB signaling is one of the prime regulators of B cell proliferation, differentiation and (auto)antibody production. Moreover, NF-κB activation leads to pro-inflammatory cytokine and chemokine production. JAK-STAT signaling is induced after activation of the B cell receptor (BCR) and is also involved in B cell proliferation and maturation. Targeting NF-κB or JAK-STAT signaling may advance our understanding of the mechanisms involved in the activation of ACPA-producing B cells. We make use of ACPApos B cell clones, since peripheral ACPApos cells are present at low frequencies in the peripheral blood.
We aimed to identify whether NF-κB or JAK-STAT signaling inhibition using small molecule inhibitors (SMIs) is effective in targeting functional responses of ACPApos B cell clones from RA patients.
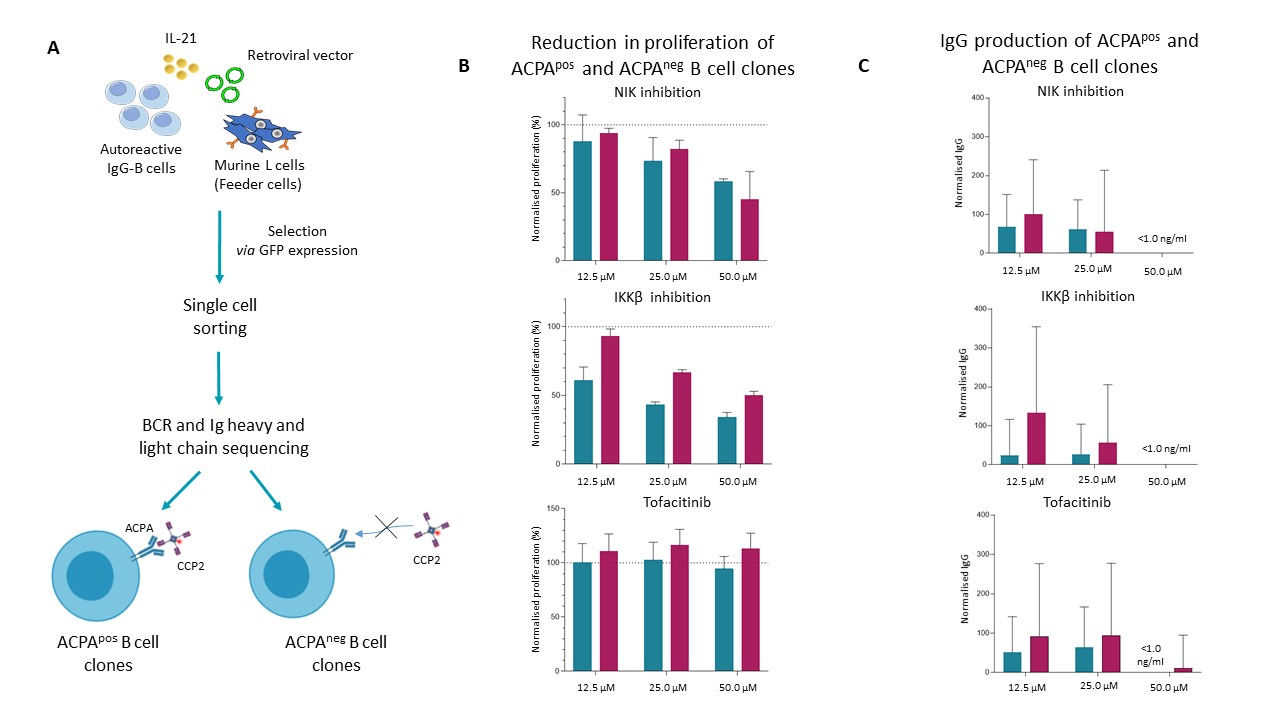
Methods: Previously generated ACPApos and ACPAneg B cell clones (Fig. 1A) were expanded and cultured with anti-CD40 and IL-21. Canonical and non-canonical NF-κB signaling were targeted by validated SMIs of Inhibitor of κB kinase β (IKKβi, canonical NF-κB signaling) and NF-κB inducing kinase (NIKi, non-canonical NF-κB signaling), respectively. Tofacitinib (a JAK1/JAK3 specific SMI) was used to target JAK-STAT signaling. Cell viability, proliferation and differentiation were evaluated by flow cytometry. Antibody production was measured by ELISA.
Results: We observed a dose-dependent reduction in proliferation in ACPApos B cell clones treated with either IKKβi (12.5 µM IKKβi: 39.12±9.86% decrease, 25 µM IKKβi: 56.55±1.84% decrease, 50 µM IKKβi: 65.65±3.1% decrease) or NIKi (12.5 µM NIKi: 12.13±19.42% decrease, 25 µM NIKi: 26.63±17.37% decrease, 50 µM NIKi: 41.69±2.00% decrease) upon stimulation with 2.25 µg/ml anti-CD40 and 1.0 ng/ml IL-21 (Fig. 1B). Similarly, we observed a dose-dependent reduction in IgG production (Fig. 1C). IKKβi treatment seemed to have a stronger effect than NIKi treatment on ACPApos B cell clones.
We did not observe a clear difference between IKKβi and NIKi in ACPAneg B cells (12.5 µM IKKβi: 6.90±5.38% decrease, 25 µM IKKβi: 33.32±2.15% decrease, 50 µM IKKβi: 50.11±3.15% decrease; 12.5 µM NIKi: 6.09±3.61% decrease, 25 µM NIKi: 17.83±6.53% decrease, 50 µM NIKi: 54.67±20.37% decrease). Cell viability was not affected by IKKβi or NIKi treatment. In contrast to IKKβi and NIKi treatment, tofactinib only had limited effects on ACPApos and ACPAneg B cell proliferation and IgG production. At present these results are being corroborated in freshly isolated ACPApos B lineage cells of RA patients.
Conclusion: Our data point towards a critical role of the NF-κB signaling pathways in the functional responses of ACPA-producing B cells, whereas a limited role of JAK-STAT signaling was observed. Consequently, targeting NF-κB signaling may have beneficial effects in limiting (autoreactive) B cell responses in RA.
To cite this abstract in AMA style:
Frazzei G, van Hamburg J, van Vollenhoven R, Tas S. NF-κB Signaling Is Critically Important for Functional Responses of ACPA-producing B Cell Clones from Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/nf-%ce%bab-signaling-is-critically-important-for-functional-responses-of-acpa-producing-b-cell-clones-from-patients-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/nf-%ce%bab-signaling-is-critically-important-for-functional-responses-of-acpa-producing-b-cell-clones-from-patients-with-rheumatoid-arthritis/