Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Clinical trial (CT) participation is essential for the advancement of treatment paradigms and should be considered part of clinical care in SLE. CTs fail to reflect the racial and ethnic diversity of SLE patients. Historically, minority groups host negative attitudes towards clinical research, in part, due to a lack of knowledge about CT protections. Accordingly, this program was initiated to improve the outlook of minority patients on CTs.
Methods: This study is an SLE randomized educational intervention. The study was modeled after the Lupus Research Alliance Patient Advocates for Lupus Studies (PALS) program and provided outreach to a racially and ethnically diverse group of SLE patients from three New York City academic lupus centers. SLE patients were appraised of the study and invited to participate during routine clinic visits. After a pretest that evaluated knowledge, attitudes, self-efficacy, and intentions for participation, patients were randomized and received the SLE CT educational program. Subsequently, patients completed a posttest to assess the effectiveness of the intervention. Additionally, patient feedback was collected to assess satisfaction with the program. Statistical significance was calculated using a paired t-test for the pre- and posttests.
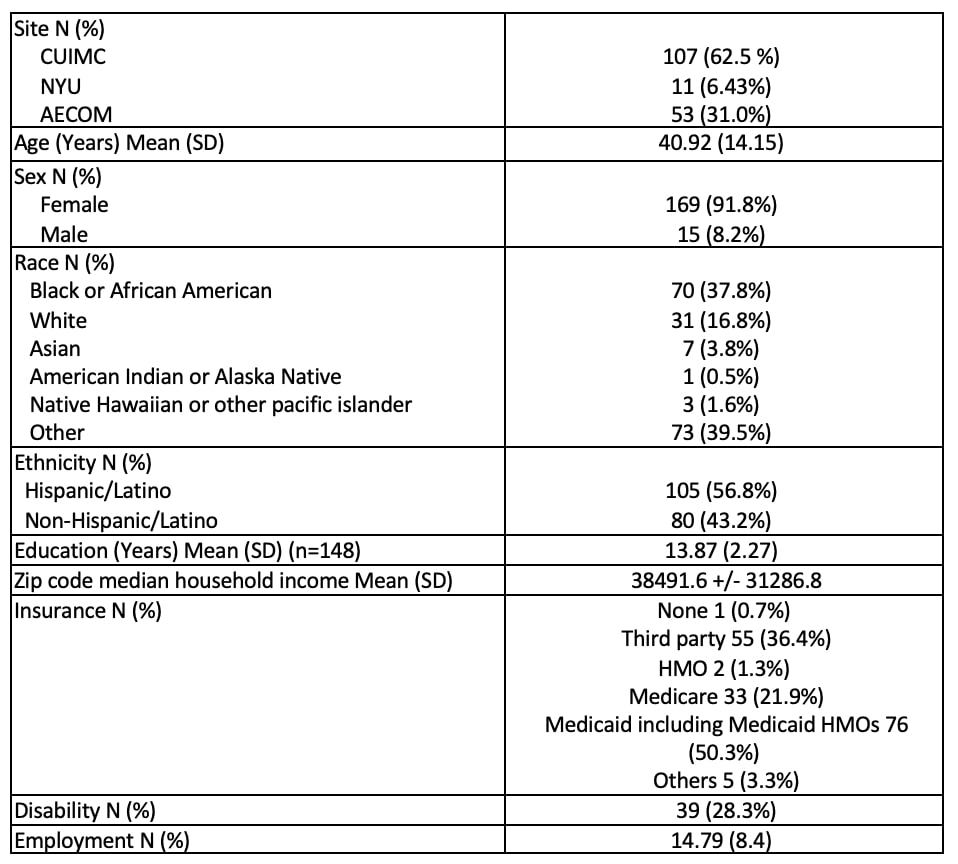
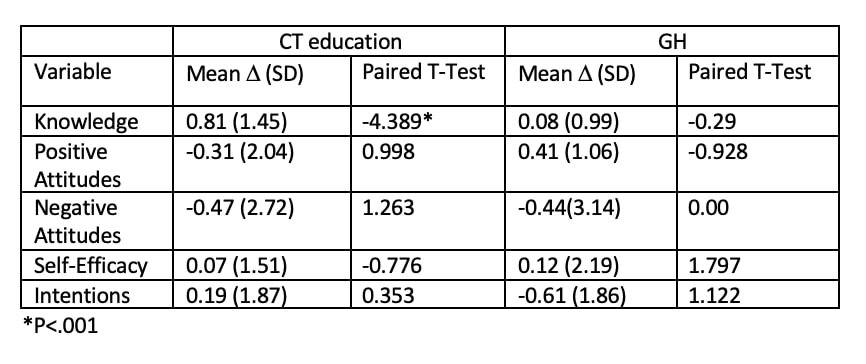
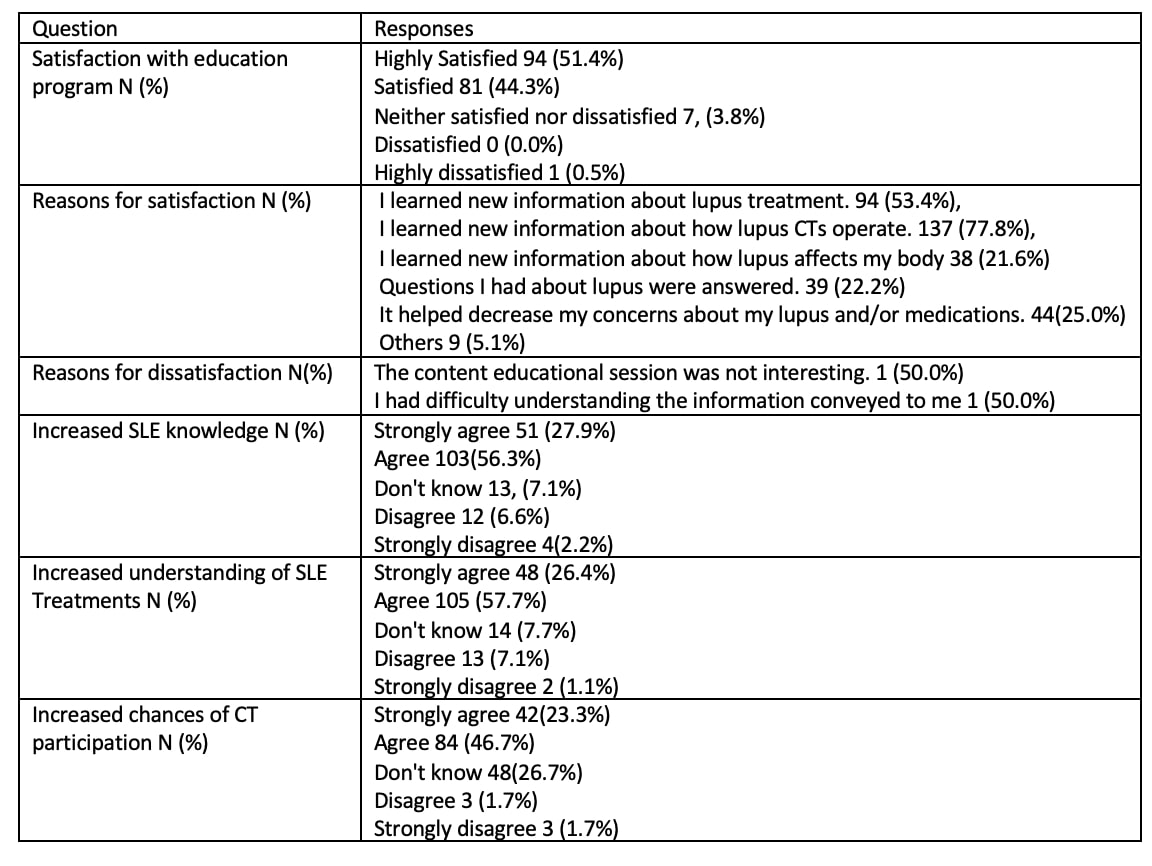
Results: 201 patients were included in the study; 10 participated in the development of a CT decision aid, previously reported. 142 patients were enrolled in the educational program, 125 actually received the CT education sessions and 49 were randomized to receive control GH education, 46 participated in these sessions. Demographic data is shown in Table 1; 105 (56.8%) identify as Hispanic and 70 (37.8%) as Black. Of the 142 subjects enrolled in the educational sessions, 62 completed the CT education and the post-test; of the 49 patients in the general education group, 13 completed the GH education sessions and the post-test. CT knowledge was assessed on a scale of 0-5; participants in the CT education program increased knowledge by an average of 0.81 points (SD 1.45). The results of the pre and post-test showed that negative attitudes towards CTs decreased on average by 0.47 points (SD 2.72) in the CT education program and by 0.44 (SD 3.14) in the control arm (Table 2). Additionally, on the paired t-test, self-efficacy showed a slight increase in the CT education program. As shown in Table 3, 126 (70%) of patients self-reported that they were more likely to participate in CTs. 94.5% of patients reported satisfaction with their participation in the program, reasons for satisfaction are shown in Table 3.
Conclusion: These data suggest that the educational program was effective at increasing patient understanding of SLE CTs. Education alone showed a trend towards altering attitudes, self-efficacy, or intentions. Patients provided positive feedback about the education they received and improved attitudes towards CTs after this program. Further research is needed to better understand how to most effectively increase minority participation in CTs.
To cite this abstract in AMA style:
Souvignier M, Khalili L, Fu W, Geraldino L, Schmidt N, Gartshteyn Y, Izmirly P, Schwartz N, Askanase A. New York City Lupus Clinical Trials Education Program [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/new-york-city-lupus-clinical-trials-education-program/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/new-york-city-lupus-clinical-trials-education-program/