Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Systemic sclerosis (SSc) is associated with a wide range of symptoms and significant impairments in function and quality of life. The goal of the Clinical Outcome Assessments for Systemic Sclerosis Clinical Trials (COAST) Project was to construct robust SSc-specific, patient-reported outcome (PRO) measures for early SSc clinical trials.
Methods: We constructed the COAST Candidate Measures in a 6-step process: 1) Conducted qualitative concept elicitation interviews (CEIs) in patients with SSc, 2) Conducted concept mapping analysis to identify conceptual domains that emerged from the CEI data, 3) Prioritized concepts and identified conceptual gaps by conducting mixed methods analysis and leveraging SSc disease expert input, 4) Developed the item pool by utilizing existingresources (HealthMeasures®, FDA Voice of the Patient Report, available SSc-specific measures), 5) Reduced the item pool through clinical and research expert review, and 6) Constructed the candidate SSc PRO measures.
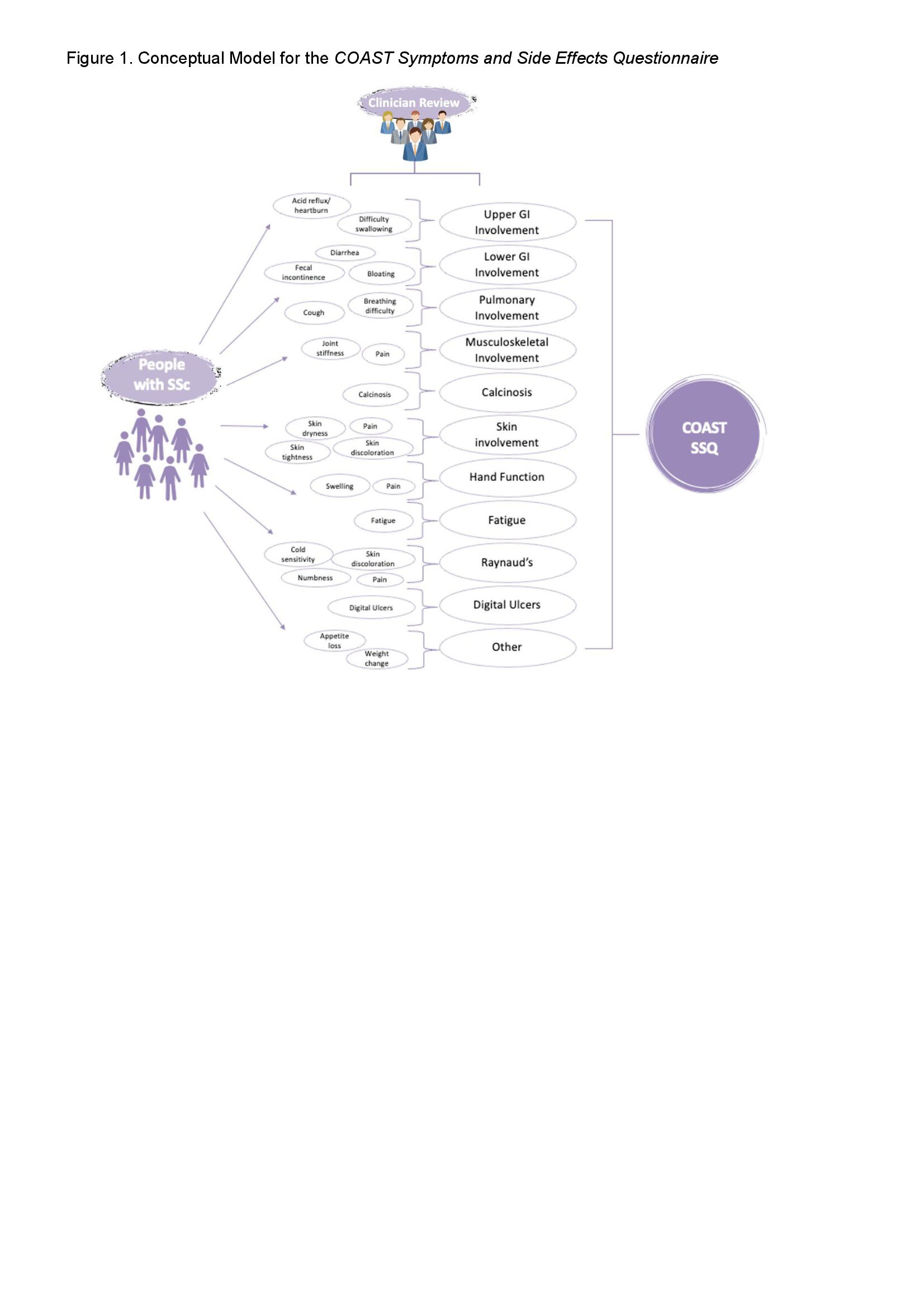
Results: 42 participants (21 each with limited cutaneous SSc (lcSSc) and diffuse cutaneous (dcSSc)) with disease duration of < 7 years (from first non-Raynaud’s phenomenon sign or symptom) were recruited at a scleroderma clinic. This sample size was sufficient to elicit a comprehensive list of symptoms, treatment side effects (TSEs), and impacts for each patient group and achieve thematic saturation. Participants reported 76 symptoms/TSEs (or concepts). Concepts identified as not a SSc symptom or TSEs by expert review were dropped and a total of 52 unique symptoms/TSEs were mapped into 16 domains. Of these 52 concepts, 34 (65.4%) were reported by participants representing both subtypes of SSc; 13 (25.0%) and 5 (9.6%) were reported only by participants with dcSSc and lcSSc, respectively. We mapped these concepts to existing items from PROMIS item banks, FACIT item banks, the draft CRISTAL measure (in development), and the SSPRO measure. We selected the items that would be considered most proximal to a PRO endpoint model as comprising the primary clinical trial instrument. This instrument is considered as a measure of the most likely direct effects of treatment, with its focus on SSc symptoms and TSEs. The items set aside earlier were found to focus on symptom impacts, physical function, and general quality of life concerns. Two candidate measures were therefore produced: COAST Symptoms and Side Effects Questionnaire (v1.0) is a 33-item candidate measure covering 11 domains and 27 concepts for clinical trials (Figure 1);COAST Function and Life Quality Questionnaire (v1.0) is a 20-item candidate measure covering 8 domains and 17 concepts as a complementary measure.
Conclusion: We developed preliminary COAST questionnaires for use as outcome measures in clinical trials. The next step in the process is cognitive debriefing to ensure their clarity and intended meaning, followed by the assessment of psychometric properties in a prospective cohort.
To cite this abstract in AMA style:
Khanna D, greene G, Perschon C, Lescoat A, Jaeger E, murphy S, Cella D. New Patient-Reported Outcome Measures for Early Systemic Sclerosis Using the FDA Guidance on Patient Reported Outcome Measures [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/new-patient-reported-outcome-measures-for-early-systemic-sclerosis-using-the-fda-guidance-on-patient-reported-outcome-measures/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/new-patient-reported-outcome-measures-for-early-systemic-sclerosis-using-the-fda-guidance-on-patient-reported-outcome-measures/