Session Information
Date: Tuesday, October 28, 2025
Title: (2377–2436) Systemic Lupus Erythematosus – Diagnosis, Manifestations, & Outcomes Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by dysregulation of the adaptive and innate immune systems. Neutrophils, key players in innate immunity, significantly contribute to SLE pathogenesis. This study aimed to identify transcriptomic differences in neutrophils from SLE patients and healthy individuals, analyze ex vivo adaptation in gene expression dynamics, and evaluate the impact of chimeric antigen receptor (CAR)-T cell therapy on neutrophil transcriptomic profiles.
Methods: We utilized negative selection for neutrophil isolation from seven SLE patients and three healthy individuals. RNA sequencing was performed to assess transcriptomic differences between neutrophils from SLE and healthy individuals, ex vivo transcriptional dynamics over 60 minutes, and responses to lipopolysaccharide (LPS) stimulation. Additionally, longitudinal transcriptomic data from an SLE patient undergoing KYV-101 anti-CD19 CAR-T cell therapy were evaluated.
Results: We identified 258 differentially expressed genes (DEGs) consistently distinguishing SLE neutrophils from healthy controls regardless of the ex vivo culture duration; these genes spanned multiple clusters, enriched in upregulation of interferon-related and DNA damage repair genes, and downregulation of ribosomal protein genes. Differential expression (DE) analysis for ex vivo adaptation revealed shared activation pathways, such as NF-κB and apoptosis, across time in neutrophils from both patients and healthy individuals. LPS stimulation highlighted overlapping inflammatory responses, demonstrating retained functional capacities in SLE neutrophils. Following CAR-T cell therapy of an SLE patient, the neutrophil transcriptomic profile realigned with healthy control characteristics, with most gene clusters approaching normalcy by three months post-treatment.
Conclusion: Neutrophils in SLE exhibit intrinsic, disease-specific transcriptomic alterations but also share certain ex vivo adaptation dynamics with healthy individuals across diverse biological pathways. Notably, anti-CD19 CAR-T cell therapy demonstrates a profound ability to reset neutrophil gene expression toward healthy patterns by three months post-treatment, despite targeting B cells rather than neutrophils directly. These findings provide insights into SLE pathogenesis and highlight potential therapeutic strategies targeting both adaptive and innate immunity.
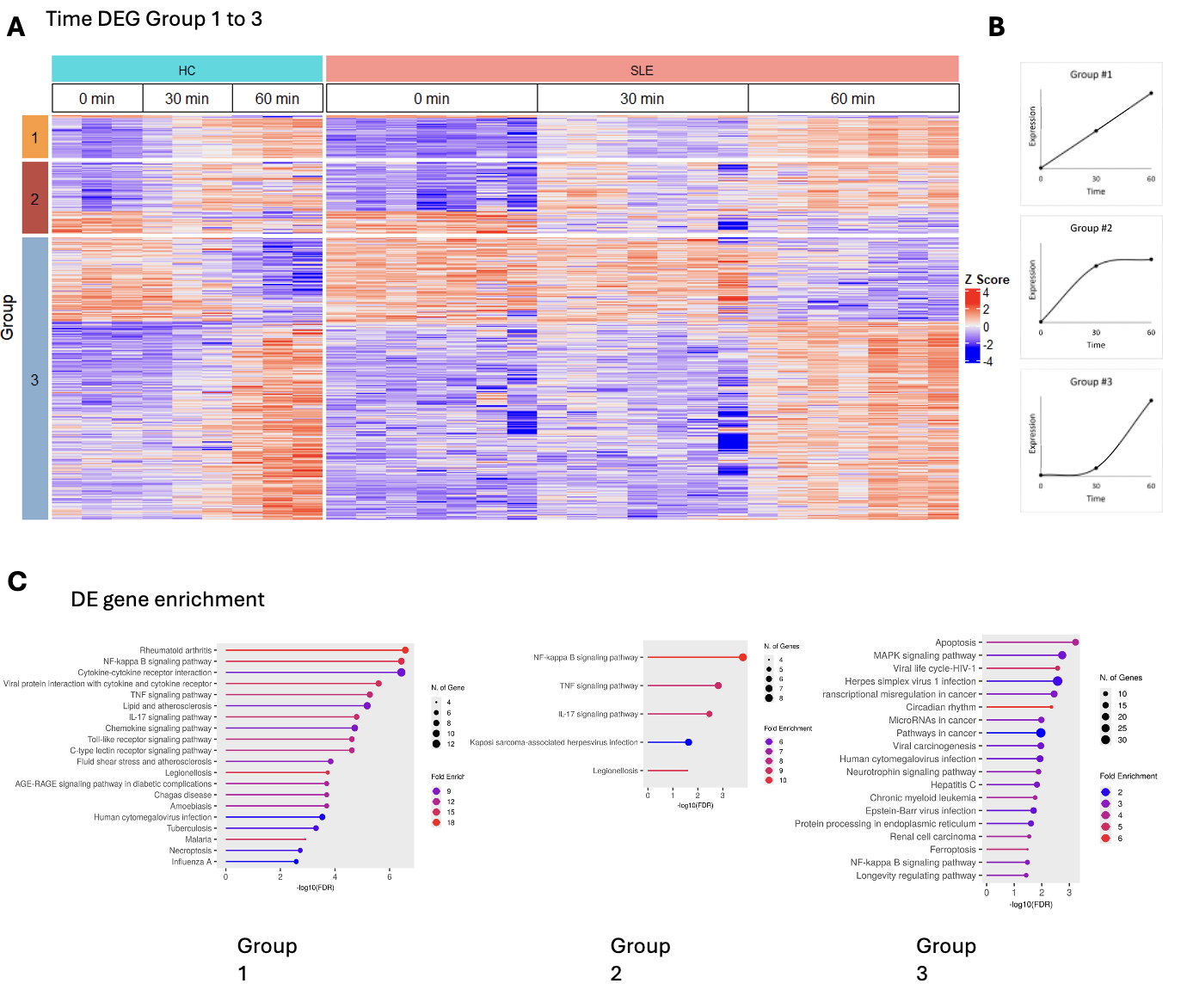 Heatmap of temporal transcriptomics in neutrophils and pathway enrichment analysis. (A) Heatmap displaying temporal transcriptomics of neutrophils categorized into three groups:(1) sustained changes from time 0 to 60 minutes, (2) changes from time 0 to 30 minutes followed by stabilization, and (3) changes only between 30 and 60 minutes. (B) Graphic Conceptualization of the 3 groups. (C) KEGG pathway enrichment analysis identifies key pathways impacted by time progression, including NF-κB activation, TNF signaling, and apoptosis, in neutrophils from both groups.
Heatmap of temporal transcriptomics in neutrophils and pathway enrichment analysis. (A) Heatmap displaying temporal transcriptomics of neutrophils categorized into three groups:(1) sustained changes from time 0 to 60 minutes, (2) changes from time 0 to 30 minutes followed by stabilization, and (3) changes only between 30 and 60 minutes. (B) Graphic Conceptualization of the 3 groups. (C) KEGG pathway enrichment analysis identifies key pathways impacted by time progression, including NF-κB activation, TNF signaling, and apoptosis, in neutrophils from both groups.
.jpg) Clustered heatmap of intrinsic DEGs. Heatmap showing expression patterns of intrinsic DEGs categorized into seven clusters including interferon-related, Rho GTPase, cytoskeleton, DNA damage repair, ribosome, and long non-coding RNA (lncRNA). The “other” cluster includes genes with no clear pathway enrichment.
Clustered heatmap of intrinsic DEGs. Heatmap showing expression patterns of intrinsic DEGs categorized into seven clusters including interferon-related, Rho GTPase, cytoskeleton, DNA damage repair, ribosome, and long non-coding RNA (lncRNA). The “other” cluster includes genes with no clear pathway enrichment.
.jpg) Longitudinal transcriptomic analysis post-CAR-T cell therapy. Heatmap visualizing intrinsic DEG expression in an SLE patient undergoing CAR T-cell therapy across baseline (two pre-treatment time points) and eight post-treatment time points. Samples from three healthy individuals serve as controls. Red arrowhead indicates day 0 (CAR T-cell infusion) and black arrowhead marks when the patient discontinued all immunosuppressive medications.
Longitudinal transcriptomic analysis post-CAR-T cell therapy. Heatmap visualizing intrinsic DEG expression in an SLE patient undergoing CAR T-cell therapy across baseline (two pre-treatment time points) and eight post-treatment time points. Samples from three healthy individuals serve as controls. Red arrowhead indicates day 0 (CAR T-cell infusion) and black arrowhead marks when the patient discontinued all immunosuppressive medications.
To cite this abstract in AMA style:
Dehdashtian E, Gallucci S, Hu G, Borie D, Caricchio R. Neutrophil Transcriptomics in SLE: Exploring Intrinsic, Ex Vivo Adaptation, and CAR-T Cell Therapy-Induced Changes [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/neutrophil-transcriptomics-in-sle-exploring-intrinsic-ex-vivo-adaptation-and-car-t-cell-therapy-induced-changes/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/neutrophil-transcriptomics-in-sle-exploring-intrinsic-ex-vivo-adaptation-and-car-t-cell-therapy-induced-changes/
