Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The SARS-CoV-2 virus mutates continuously, posing challenges for patients with immune-mediated inflammatory diseases (IMIDs) on tumour necrosis factor inhibitors (TNFi). These patients often have diminished vaccine responses and are at a higher risk of severe infections. The efficacy of the recommended mono- and bivalent vaccine boosters in this patient population remains unclear.
The objective of this study was to assess the neutralizing capacity and anti-spike antibody responses to SARS-CoV-2 in TNFi-treated IMID patients, after receiving a monovalent 4th vaccine dose and a bivalent 5th vaccine dose, compared to those with hybrid immunity.
Methods: These analyses from the ongoing observational Nor-vaC study included TNFi-treated IMID patients who received either a 5th vaccine dose or experienced SARS-CoV-2 infection following a 4th vaccine dose (hybrid immunity).
Blood samples were collected 2-4 weeks after the 4th monovalent vaccine dose and after either subsequent COVID-19 or a 5th bivalent dose (BA.1 or BA.4/5).
Neutralizing antibody titres against three viral variants (Wuhan (WT), Omicron BA.1, and Omicron BA.4), and IgG anti-spike antibody levels, were analysed. Group comparisons were performed with Mann Whitney U test.
Results: From December 17, 2021, to June 20, 2023, 371 IMID patients (86 rheumatoid arthritis, 72 psoriatic arthritis, 95 spondyloarthritis, 75 Crohn’s disease, 43 ulcerative colitis) on TNFi in mono- (60%) or combination (40%) therapy received a 4th and a 5th vaccine dose or had a SARS-CoV-2 infection after the 4th vaccine dose, median age 58 (IQR 48-67), 53 % female.
In total, 322 patients, received a 5th bivalent vaccine dose, either BA.1 (40%), BA.4/5 vaccine (60%). Overall, 180 (49%) had COVID-19 following a 4th vaccine dose (4th hybrid). All infections occurred while BA.1, BA.4 and BA.5 were the circulating variants.
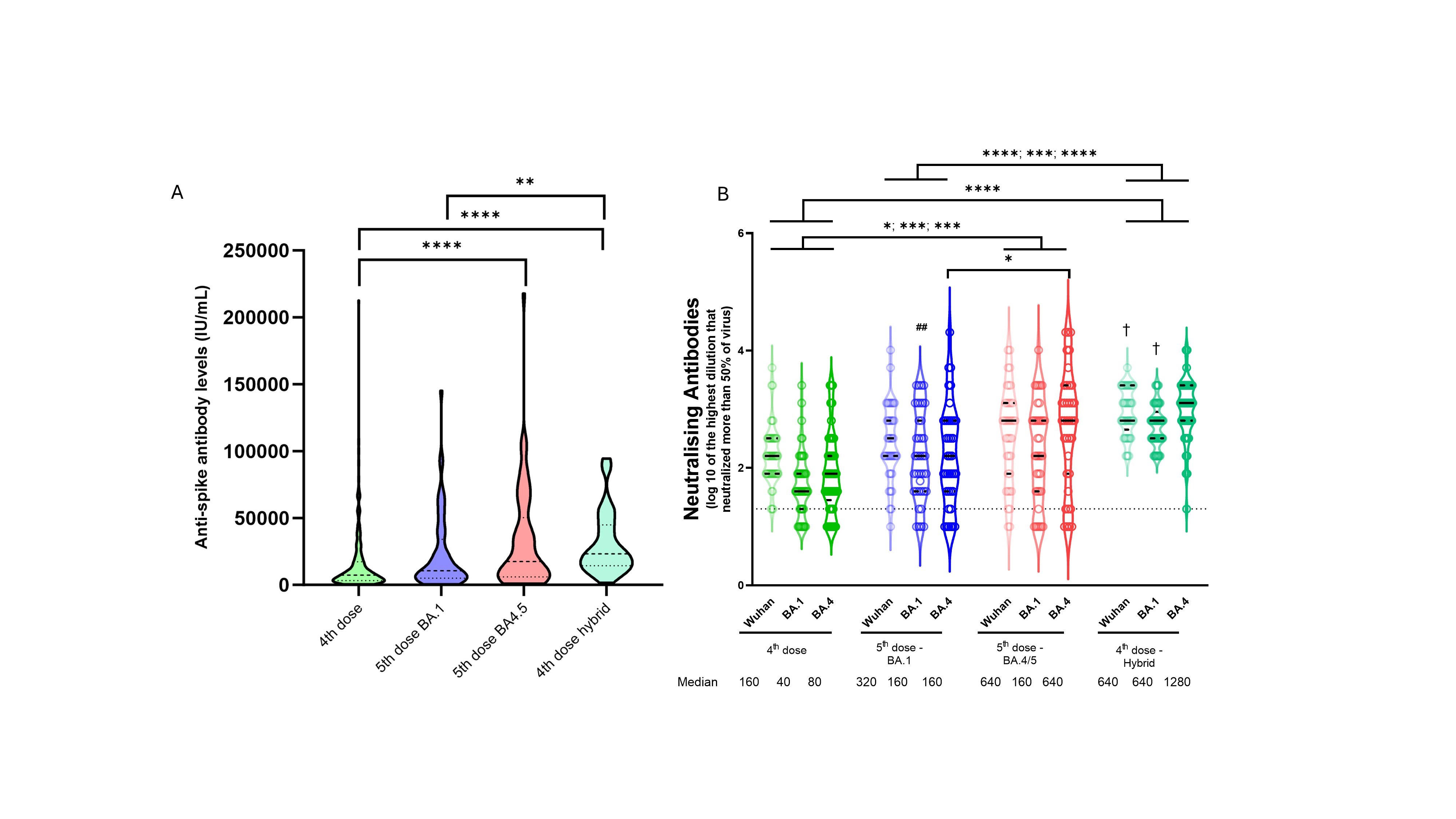
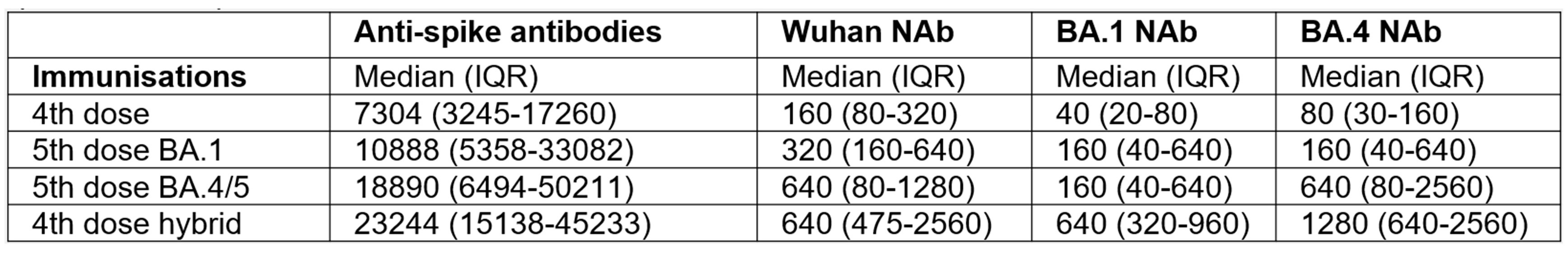
Patients with hybrid immunity had a higher median anti-spike antibody level than did infection-naïve patients following the 5th dose BA.1 vaccine (p=0.0015), but comparable to levels after a 5th BA.4/5 vaccine dose (p=0.24) (figure A, table). Hybrid immunity increased neutralizing antibody titres against all tested variants compared to a 4th vaccine dose (WT: p< 0.0001), BA1: p< 0.0001, BA4: p< 0.0001) and 5th vaccine dose with BA.1 (WT: p< 0.0001, BA1: p=0.0002, BA4: p< 0.0001) (figure B, table).
In infection-naïve patients, a 5th BA.4/5 vaccine increased neutralizing antibody titres against all viral variants tested (WT, BA.1 and BA.4), compared to post-4th dose levels (WT: p=0.011, BA.1: p=0.001, BA.4: p=0.0001) (figure B, table). In infection-naïve patients, a 5th BA.4/5 vaccine gave higher neutralizing antibody responses against BA.4 than a 5th BA.1 vaccine (p=0.023) and comparable to that after hybrid immunity (p=0.16) (figure B, table).
Conclusion: In IMID patients on TNFi who reported COVID-19 as a fifth antigenic exposure, neutralising antibody capacity was superior to that following a fifth updated, bivalent vaccine in patients that were infection-naïve. Infection-naïve patients benefit from the most updated vaccine, providing a humoral response almost comparable to that following infection.
To cite this abstract in AMA style:
Ørbo H, Kasahara T, Wolf A, Bjørlykke K, Sexton J, Jyssum I, Tveter A, Solum G, Kjønstad I, Lind A, Dimova-Svetoslavova V, Kvien T, Jahnsen J, Haavardsholm E, Munthe L, Provan S, Vaage J, Mjaaland S, Jørgensen K, Grødeland G, Syversen S, Goll G. Neutralising Antibody Responses to Bivalent SARS-CoV-2 Vaccines and Hybrid Immunity in Patients on TNF Inhibitors: A Prospective Cohort Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/neutralising-antibody-responses-to-bivalent-sars-cov-2-vaccines-and-hybrid-immunity-in-patients-on-tnf-inhibitors-a-prospective-cohort-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/neutralising-antibody-responses-to-bivalent-sars-cov-2-vaccines-and-hybrid-immunity-in-patients-on-tnf-inhibitors-a-prospective-cohort-study/