Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Neuroimmune modulation by electrical stimulation of the left cervical vagus nerve represents a novel treatment option for rheumatoid arthritis (RA). We present 12-month efficacy and safety outcomes for an implantable vagus nerve stimulation device to treat biologic-experienced patients with RA (the RESET-RA study, ClinicalTrials.gov, NCT04539964).
Methods: In this multicenter, randomized, double-blind, sham-controlled, pivotal trial, patients with prior inadequate response or intolerance to at least one biologic or targeted synthetic disease-modifying antirheumatic drug (b/tsDMARD) were randomly assigned to stimulation (treatment) or sham. The primary endpoint of ACR20 response was assessed 3 months after randomization. After 3 months, sham crossed over to treatment, and all patients received stimulation during open-label follow-up, with results reported through 12 months. Secondary endpoints included safety measures, disease activity scores, and use of RA drugs in combination with stimulation.
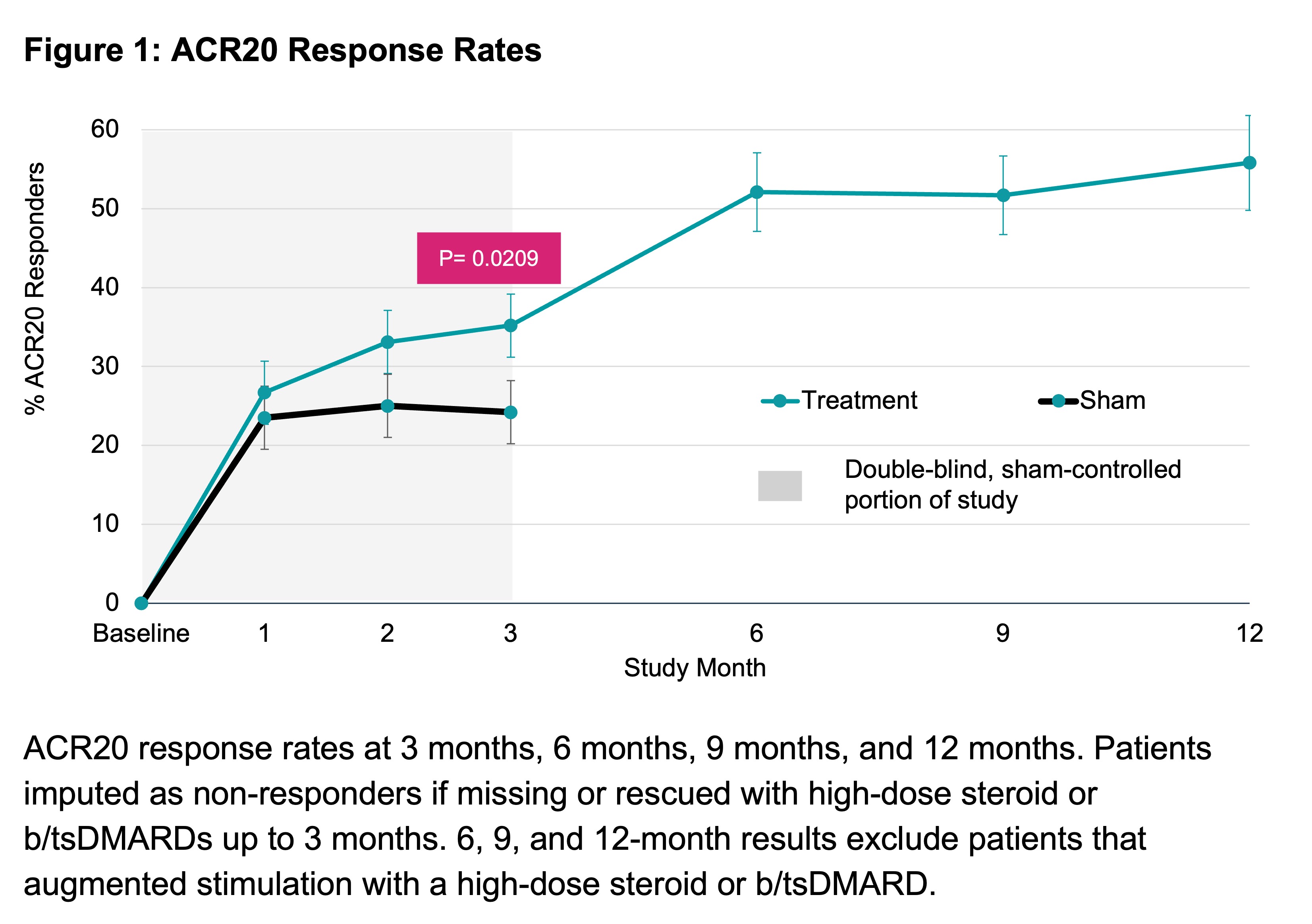
Results: 242 participants across 41 US study sites were consented and underwent device implantation followed by 1:1 randomization to treatment or sham. Baseline characteristics were well-balanced with mean age of 56 years, 86% female, RA duration 12.4 years, 15 tender joints, and 10 swollen joints (28 joint count). Overall, 39% of patients had been treated with exactly 1 prior b/tsDMARD, 22% with exactly 2, and 39% with 3 or more. 43% of all patients were exposed to b/tsDMARDs with differing mechanisms of action.At 3 months, a significantly greater percentage of patients in the treatment group were ACR20 responders (treatment 35.2% [43/122] vs. sham 24.2% [29/120], p=0.0209). Response rates continued to improve for treatment group: 52.1% at 6 months, 51.7% at 9 months, and 55.8% at 12 months (Figure 1). Disease activity scores showed a similar trend with 49.3% attaining DAS28-CRP low disease activity or remission, 47.4% attaining CDAI low disease activity or remission and 77.3% attaining EULAR good/moderate response at 12 months (Figure 2). Augmentation of stimulation therapy with a b/tsDMARD was allowed without restriction following the 3-month, controlled portion of participation. By 12 months, only 24.8% of patients added a b/tsDMARD to stimulation therapy and 97.5% of all patients remained persistent with stimulation therapy. The implant procedure was well-tolerated, with a serious adverse rate of 1.7%, all occurring during the perioperative period. The most common, related adverse event was transient vocal cord paresis presenting as hoarseness following the implant procedure. Stimulation was well-tolerated, with few non-serious events, such as pain or sleep disturbance. These were typically managed by adjusting the stimulation dose. No deaths or unanticipated adverse device effects occurred (Table 1).
Conclusion: RESET-RA met its primary efficacy and safety endpoints, demonstrating that vagus nerve-mediated neuroimmune modulation by electrical stimulation using an implantable device is well-tolerated with durable efficacy through 12-months among adults with active RA disease and inadequate response or intolerance to at least one b/tsDMARD.
To cite this abstract in AMA style:
Tesser J, Crowley A, Box J, June J, Wickersham P, Valenzuela G, Gaylis N, Lam G, Pacheco L, Ridley D, Pinto-Patarroyo G, Novack S, Churchill M, Kohler M, Lee E, Pando J, Parris G, Peterson J, Shah T, Singhal A, Vuong V, Curtis J, Chernoff D. Neuroimmune modulation for the Treatment of Rheumatoid Arthritis: Results at 12 months from a Randomized, Sham-Controlled, Double-Blind Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/neuroimmune-modulation-for-the-treatment-of-rheumatoid-arthritis-results-at-12-months-from-a-randomized-sham-controlled-double-blind-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/neuroimmune-modulation-for-the-treatment-of-rheumatoid-arthritis-results-at-12-months-from-a-randomized-sham-controlled-double-blind-study/

.jpg)
.jpg)