Background/Purpose: Anti-citrullinated protein antibodies (ACPAs) are characteristic of rheumatoid arthritis however, their presence years before onset of clinical RA is perplexing. Although multiple putative citrullinated antigens have been identified, including citrullinated products of NETosis, no studies have demonstrated the capacity of these antigens to initiate inflammatory arthritis. We sought to identify citrullinated products of NETosis targeted by the RA immune response and with the capacity to drive inflammatory arthritis.
Methods: We performed proteomic analysis of human NETs to identify all citrullinated proteins including those targeted as part of the RA immune response. Using a combination of ELISA and IHC we compared RA and OA serum, synovial fluid and synovial tissue for levels of histone 2B (H2B), anti-H2B antibodies, as well as H2B-containing immune complexes. Using macrophage activation assays we assessed the effect of histone citrullination on immunostimulatory capacity and evaluated the stimulatory capacity of native and citrullinated H2B-containing immune complexes. Finally, we immunized mice with citrullinated H2B (cH2B) with and without the induction of low grade collagen induced arthritis to assess the potential for anti-cH2B antibodies to mediate arthritis in vivo.
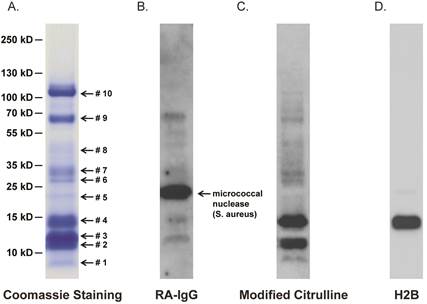
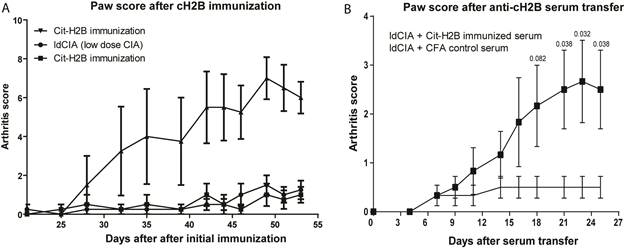
Results: Proteomic interrogation of NET-derived proteins, RA serum, synovium, and synovial fluid identified robust targeting of NET-derived citrullinated histones by the ACPA immune response. Over 90% of RA patients have anti-cH2B antibodies and over half have measurable levels of synovial fluid H2B immune complexes. We observe that histone citrullination increases innate immunostimulatory capacity and that immune complexes containing citrullinated histones both activate macrophage cytokine production and propagate NETosis. Finally, we demonstrate that autoimmunity to cH2B is arthritogenic, both by primary immunization as well as immune serum transfer, but only in the setting of underlying low grade articular inflammation.
Conclusion: We identify cH2B as an antigenic target of the ACPA immune response and our findings suggest that intra-articular histone citrullination can link innate immunity via NETosis and adaptive immunity via generation of citrullinated histone immune complexes. The generation of citrullinated histone antigens during low grade articular inflammation provides a potential mechanism for the conversion from asymptomatic ACPA seropositivity to clinical RA.
Disclosure:
D. H. Sohn,
None;
K. Onuma,
None;
C. Rhodes,
None;
X. Zhao,
None;
T. Gazitt,
None;
R. Shiao,
None;
J. Fert Bober,
None;
D. Cheng,
None;
L. J. Lahey,
None;
H. Wong,
None;
J. van Eyk,
None;
W. H. Robinson,
None;
J. Sokolove,
None.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/netosis-induced-histone-citrullination-facilitates-onset-and-propagation-of-rheumatoid-arthritis/