Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Antiphospholipid syndrome (APS) and systemic lupus erythematosus (SLE) are characterized by heterogenous clinical presentations, driven by autoantibodies; patients with SLE develop APS more often than the reverse. The aim of this study was to determine the prevalence and clinical associations of SLE autoantibodies in antiphospholipid antibody (aPL)-positive patients without other systemic autoimmune rheumatic diseases (SARDs).
Methods: A web-based data capturing system is used to store patient clinical information and registry inclusion criteria are positive aPL (Revised Sapporo APS Classification Criteria), tested at least twice within one year prior to enrollment. Earliest available sera from North and South American registry patients with or without APS classification, but with no other SARDs (primary aPL/APS), were tested for IgG, IgM, IgA anticardiolipin (aCL) and anti-β2glycoprotein I (aβ2GPI) antibodies as well as anti-nuclear antibody (ANA), anti-dsDNA, anti-SSA, anti-SSB, anti-Sm, and anti-RNP antibodies using Phadia EliA® fluorescence enzyme immunoassays (Thermofisher). Autoantibody prevalence and titers were compared in three groups: 1) patients with microvascular (aPL nephropathy, livedoid vasculopathy, and/or diffuse alveolar hemorrhage) and/or hematologic disease (immune thrombocytopenia and/or autoimmune hemolytic anemia) with/without thrombotic APS (TAPS) or obstetric APS (OAPS); 2) TAPS and/or OAPS but no microvascular or hematologic disease; and c) all aPL-positive patients not included in groups 1 and 2. The association of lupus antibodies with baseline clinical and laboratory characteristics was evaluated through pairwise univariate analyses.
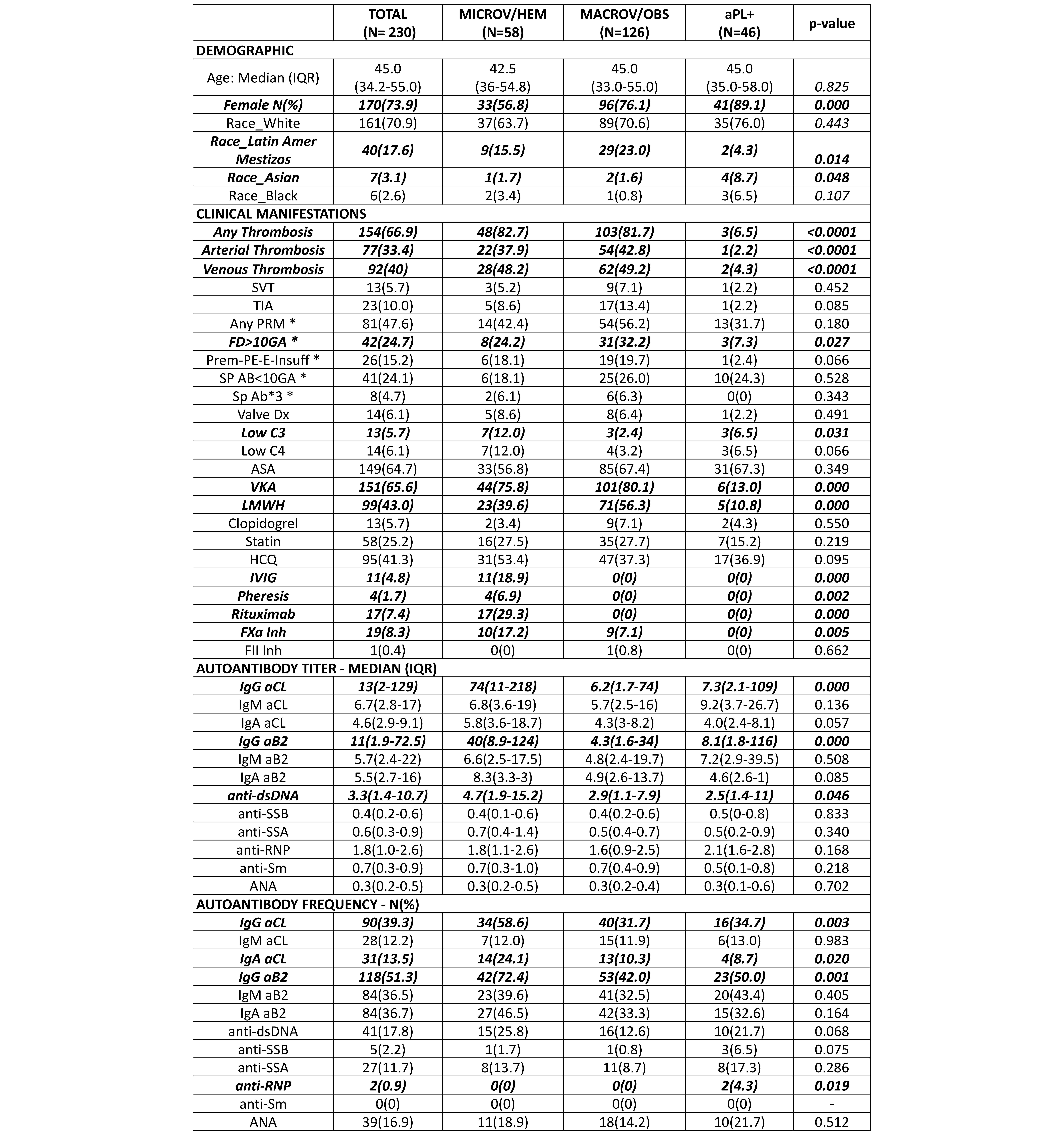
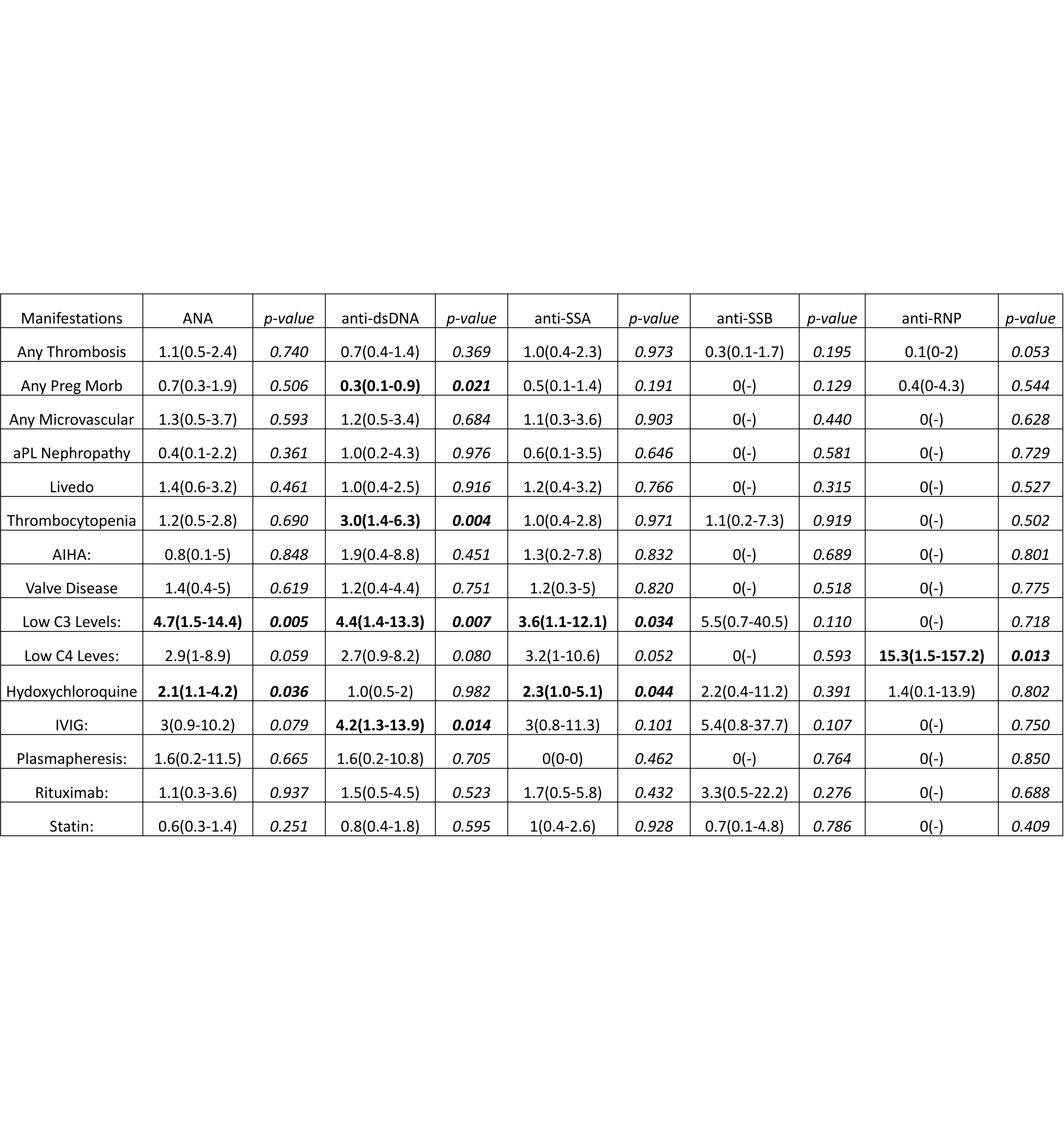
Results: Of 230 patients included (median age at registry entry 45.0 interquartile range (IQR) 34.2-55.0 years, 73.9% female) (Group 1: 58 patients, Group 2: 126, and Group 3: 46), SLE antibody prevalence ranged from 0 to 18%: anti-dsDNA 41 (18%), ANA 39 (17%), anti-SSA 27 (12%), anti-SSB 5 (2%), anti-RNP 2 (1%), and anti-Sm 0 (0%) (Table 1). Anti-dsDNA titers, and the prevalence and titers of IgG aCL and IgG aβ2GPI were significantly higher in patients with microvascular and/or hematologic disease. Clinical associations of SLE autoantibodies were: a) anti-dsDNA positivity with immune thrombocytopenia (odds ratio [OR] 95%CI: 3.0 [1.4-6.3], p=0.004), low C3 levels (OR 4.4 [1.4-13.3], p=0.007), and IVIG use (ever) (OR 4.2 [1.3-13.9], p=0.014); b) ANA positivity with low C3 (OR 4.7 [1.5-14.4], p=0.005) and hydroxychloroquine (HCQ) use (ever) (OR 2.1 [1.1-4.2], p=0.036); and c) anti-SSA positivity with low C3 (OR 3.6 [1.1-12.1], p=0.034) and HCQ use (OR 2.3 [1.0-5.1], p=0.044).
Conclusion: In primary aPL-positive patients with or without APS, the prevalence of SLE autoantibodies ranged from 0% to 18%, with anti-dsDNA, ANA and anti-SSA being most frequent and anti-Sm absent. Anti-dsDNA titers as well as IgG aCL/aβ2GPI levels and prevalence were significantly higher in patients with microvascular and/or hematologic disease, compared to other aPL-positive patients. Low complement values, immune thrombocytopenia, HCQ, and IVIG use in primary aPL-positive patients were associated with at least one SLE autoantibody.
To cite this abstract in AMA style:
Willis R, Tebo A, Skeith L, Kello N, Belmont H, Fortin P, Ramires de Jesús G, Branch D, Petri M, Knight J, Kose Cobanoglu R, Bertolaccini M, Cohen H, Roubey R, Erkan D, Andrade D. Natural History and Clinical Implications of Lupus Autoantibodies in Primary Antiphospholipid Antibody Positive Patients: Results from the AntiPhospholipid Syndrome Alliance for Clinical Trials and InternatiOnal Networking (APS ACTION) Clinical Database and Repository (“Registry”) [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/natural-history-and-clinical-implications-of-lupus-autoantibodies-in-primary-antiphospholipid-antibody-positive-patients-results-from-the-antiphospholipid-syndrome-alliance-for-clinical-trials-and-in/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/natural-history-and-clinical-implications-of-lupus-autoantibodies-in-primary-antiphospholipid-antibody-positive-patients-results-from-the-antiphospholipid-syndrome-alliance-for-clinical-trials-and-in/