Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Predicting relapse is a major challenge in granulomatosis with polyangiitis (GPA). We previously demonstrated changes in the nasal microbiota, particularly Corynebacterium tuberculostearicum, prior to relapse in GPA. Given the important function of the nasal epithelium in regulating immune responses and host-commensal interactions, we performed longitudinal molecular profiling of the nasal epithelium to examine transcriptional changes preceding relapse in GPA.
Methods: Samples of nasal epithelial cells were longitudinally collected by nasal cytology brushes from patients with GPA and healthy controls. Pre-relapse and relapse visits from patients with GPA (“relapsing GPA”) were matched by immunosuppressive therapy to patients in stable remission (“non-relapsing GPA”). Relapse was defined as BVAS-WG > 0. We performed bulk RNA-sequencing to examine differentially expressed genes. Genes were mapped to single cell RNA-sequencing (scRNA-seq) data from nasal epithelium of healthy patients and histopathology of sinonasal biopsies were reviewed. We investigated the association between C. tuberculostearicum abundance and gene expression between relapsing vs non-relapsing GPA.
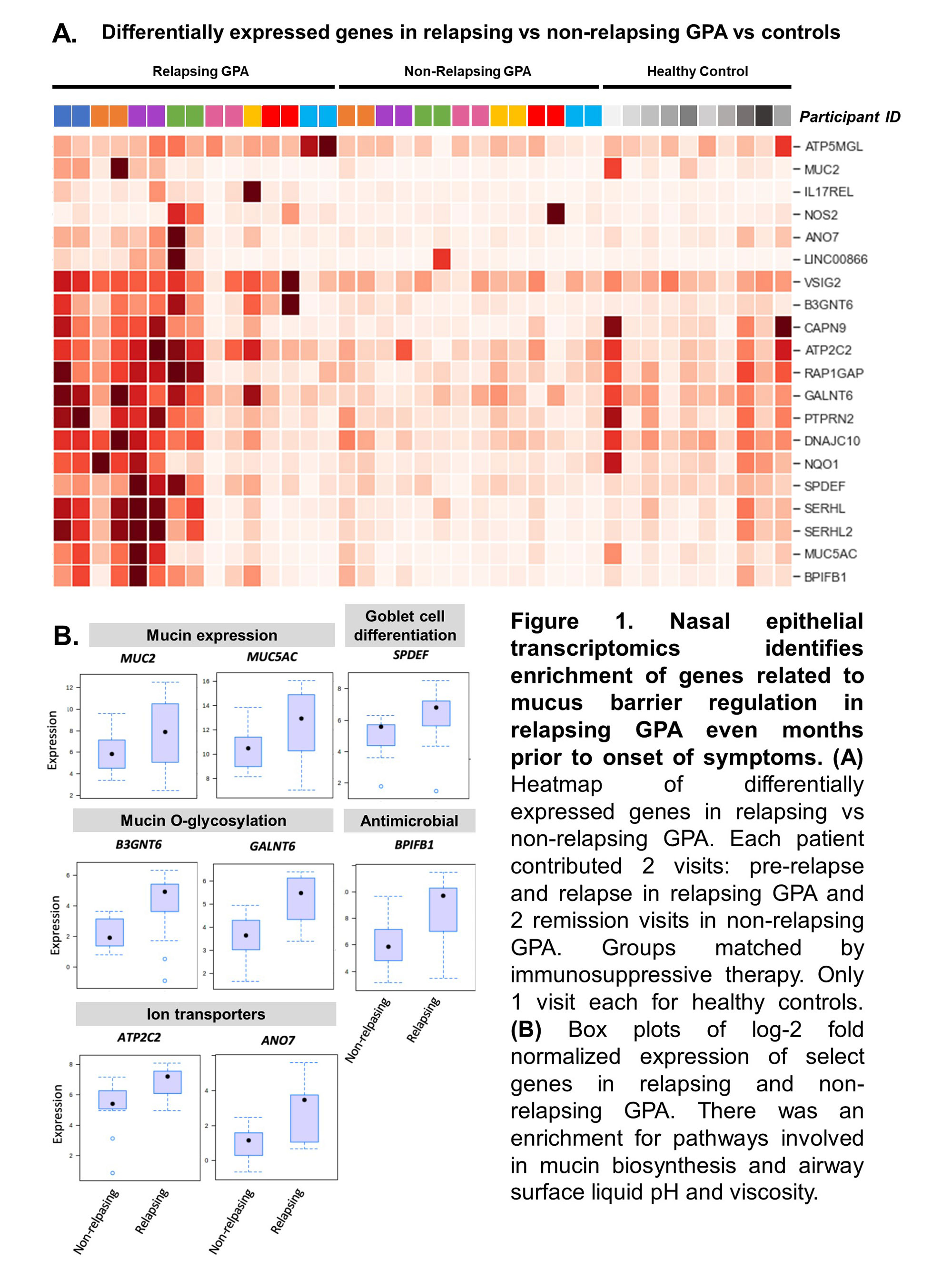
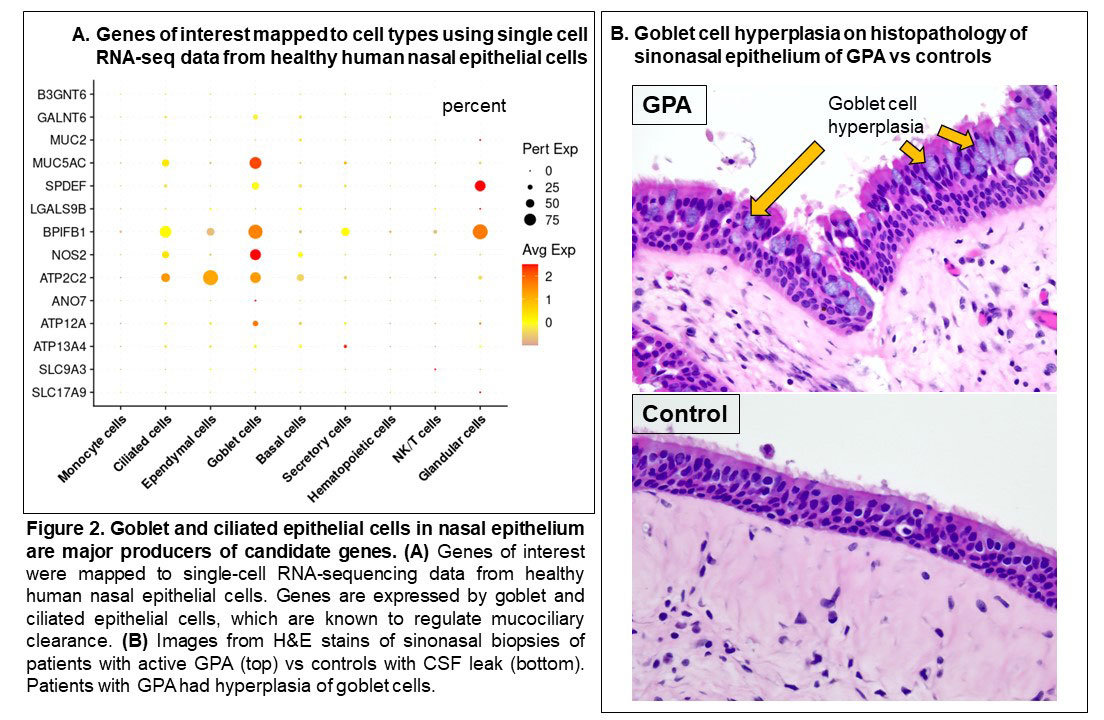
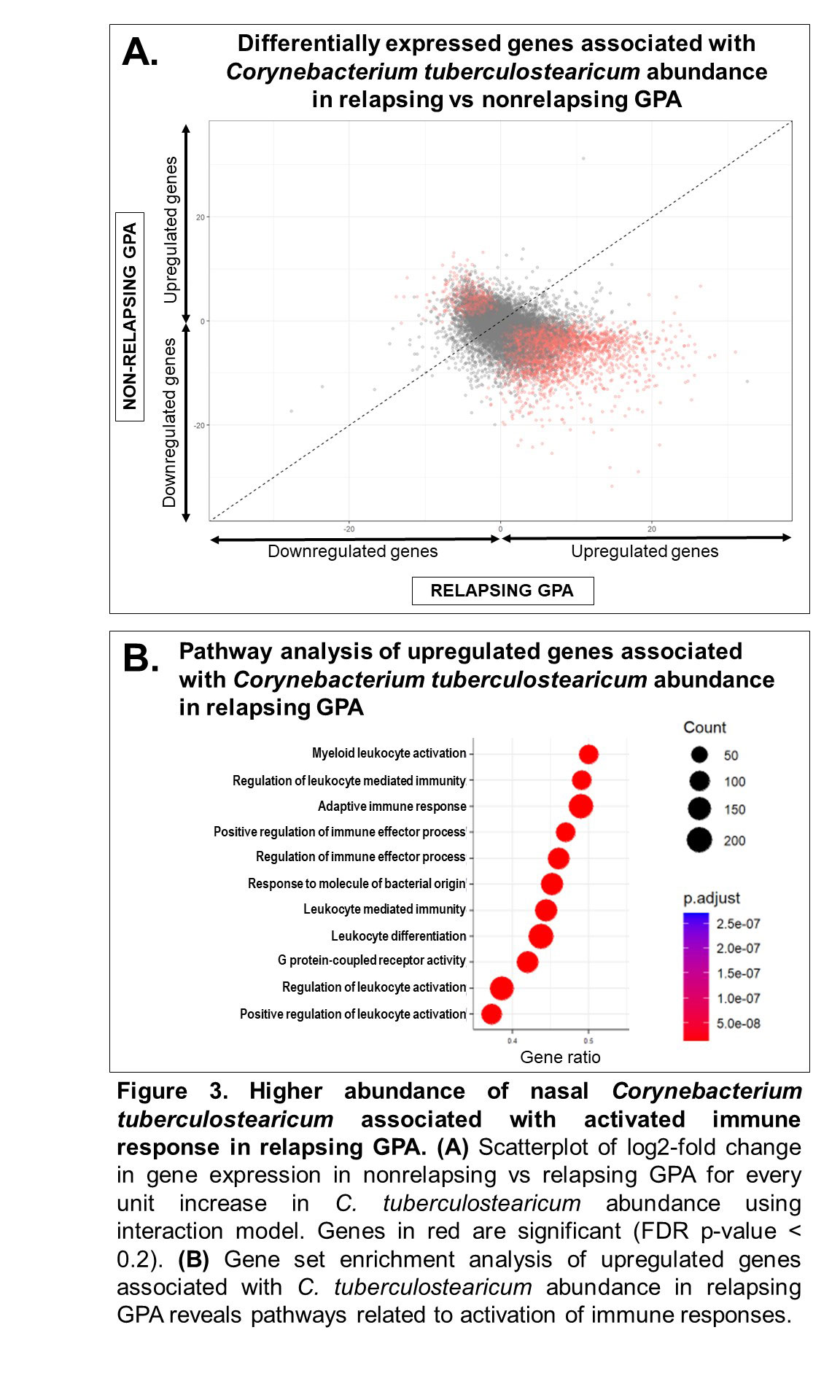
Results: The study included 39 visits: 7 pre-relapse and 8 relapse visits (8 relapsing GPA patients), 14 stable remission visits (7 non-relapsing GPA patients), and 10 healthy controls. Median months between pre-relapse and relapse visits was 6 months (IQR 4-9). There were no differences in disease duration, ANCA type, prior sinonasal involvement, Vasculitis Damage Index, or medications between the relapsing and non-relapsing GPA groups. Genes related to modulation of the mucus layer were upregulated at the pre-relapse visit months prior to onset of relapse both in patients with and without sinonasal involvement (Figure 1). When mapped to scRNA-seq data, upregulated genes were expressed by goblet and submucosal gland cells, which synthesize and secrete mucus (Figure 2A). Review of sinonasal histopathology revealed goblet cell hyperplasia in patients with active GPA vs controls (Figure 2B). Higher abundance of nasal C. tuberculostearicum was associated with genes enriched for pathways involving activation of innate and adaptive immune responses (Figure 3).
Conclusion: This study found a previously-unreported change in nasal mucus barrier regulation that is detectable several months prior to onset of relapse symptoms in GPA. These changes were detectable even in patients who did not report sinonasal symptoms at time of relapse. Furthermore, higher abundance of the nasal bacteria C. tuberculostearicum is associated with activation of immune responses in relapsing GPA. Based on these results, we propose a novel mechanism of disease for GPA in which patients with altered nasal mucus barrier are more susceptible to overgrowth of pathogenic nasal bacteria (such as C. tuberculostearicum) and their pro-inflammatory effects. These findings suggest both that minimally-invasive sampling of nasal mucosa may lead to a promising biomarker in GPA and that tailored intervention aimed at nasal microbiota could be a therapeutic target in GPA.
To cite this abstract in AMA style:
Zhao F, Deek R, Garifallou J, Jalaly J, Lin C, Reed D, Banerjee S, Amudala N, Sreih A, Chou S, Livolsi V, Collman R, Lee H, Merkel P, Grayson P, Cohen N, Miner J, Rhee R. Nasal Epithelial Gene Expression Profiling Preceding Relapse in Patients with Granulomatosis with Polyangiitis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/nasal-epithelial-gene-expression-profiling-preceding-relapse-in-patients-with-granulomatosis-with-polyangiitis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/nasal-epithelial-gene-expression-profiling-preceding-relapse-in-patients-with-granulomatosis-with-polyangiitis/