Session Information
Session Type: Abstract Session
Session Time: 5:00PM-6:00PM
Background/Purpose: Osteoarthritis (OA) represents the most common form of arthritis and is a major cause of morbidity in the aging population. Post-traumatic OA (PTOA) is a form of OA that develops after a joint injury and in which the nature and time of trauma is known. Studies have documented a robust inflammatory response in the immediate aftermath of joint injury that sets in motion a sequence of events, resulting in eventual PTOA. This inflammatory response likely contributes to chondrocyte death, impaired cartilage repair and PTOA. We have previously shown that intra-articular (IA) delivery of a nanoparticle (NP) comprising a peptide, p5RHH, complexed to siRNA suppressed NF-kB p65 expression and significantly mitigated early cartilage changes in a non-invasive joint injury model (Yan et al. Proc Natl Acad Sci USA. 2016 113:E6199-E6208). We hypothesize that suppression of the inflammatory response combined with overexpression of an anabolic factor to maintain cartilage homeostasis will mitigate pain and structural damage in a mouse model of PTOA.
Methods: We employed the amphipathic peptide p5RHH to form nanocomplexes, which have been proven safe and stable for IA delivery of both siRNA and mRNA. Mice were challenged with unilateral destabilization of the medial meniscus (DMM) to provide proof-of-concept that IA delivery of siRNA to knockdown NF-kB p65 (anti-inflammatory) combined with simultaneous delivery of mRNA to overexpress WNT16 (anabolic) will have additive effects compared to treatment with individual agent. Comparisons between groups (≥ 3) were performed by one-way ANOVA followed by Bonferroni’s post-test. P < 0.05 was considered significant.
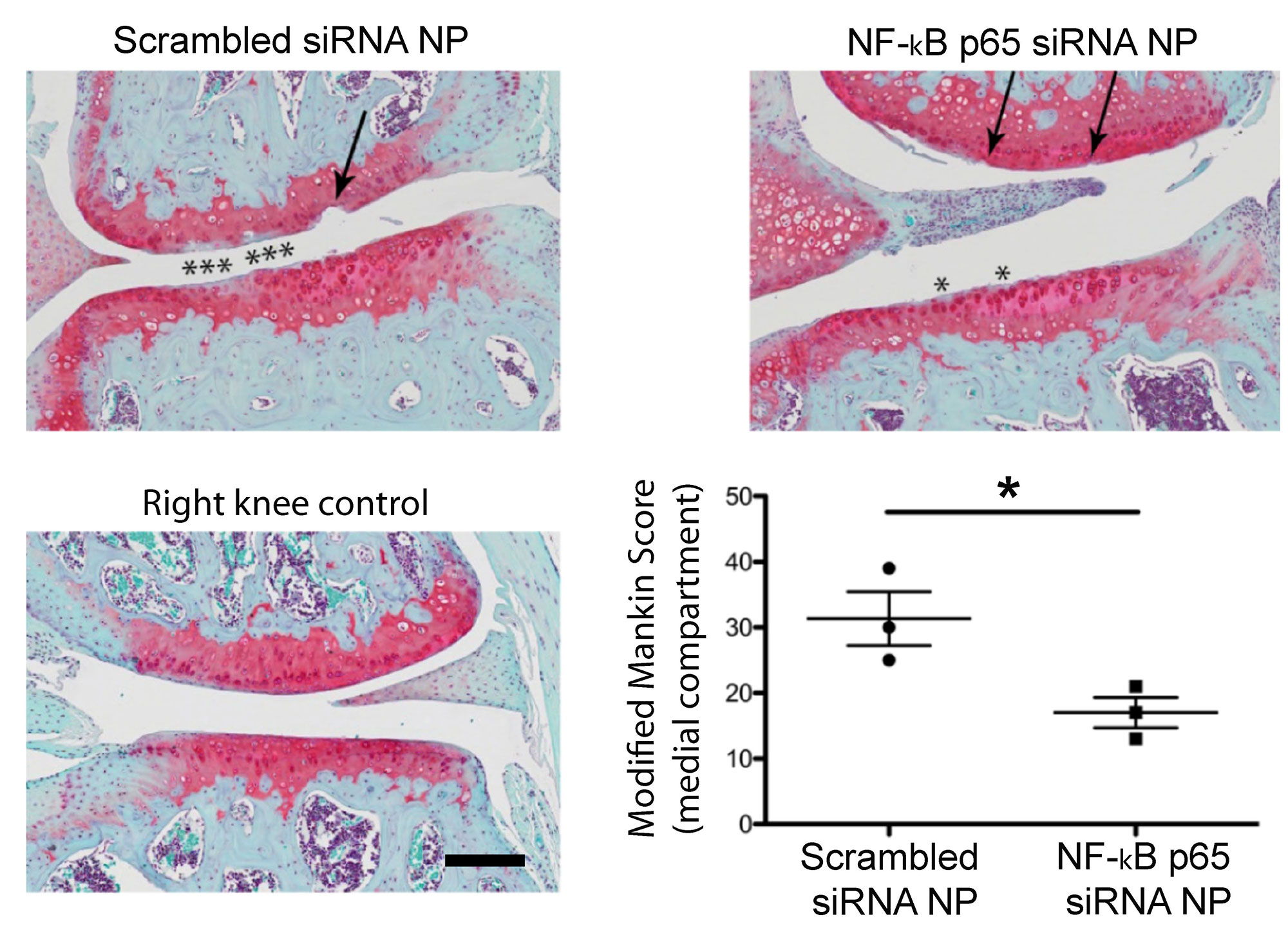
Results: Preliminary data show that p5RHH-p65 siRNA nanotherapy mitigated early DMM changes at 4 weeks, as evidenced by a lower Modified Mankin score (figure 1). We next challenged groups of mice with DMM and treated them with different NP combinations. IA treatment with a combination of p65 siRNA and WNT16 mRNA significantly reduced pressure-pain hyperalgesia in the DMM limb of both male and female mice when measured at 12 weeks post-destabilization (figure 2).
Conclusion: IA drug delivery for OA treatment hold key benefits due to increased local drug availability and reduced systemic bystander effects. However, these benefits are limited by rapid drug clearance from the joint space. We show that p5RHH-based delivery of anti-inflammatory and anabolic factors mitigates hyperalgesia, which has been shown to correlate with structural joint damage. This nanotherapeutic approach promises to overcome IA clearance deficits to halt cartilage loss and maintain cartilage homeostasis, translating to better long-term outcomes in PTOA treatment.
To cite this abstract in AMA style:
Springer L, Collins K, Yan H, Hu Y, Guilak F, Wickline S, Pan H, Pham C. Nanotherapeutic Approaches for the Treatment of Post-traumatic Osteoarthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/nanotherapeutic-approaches-for-the-treatment-of-post-traumatic-osteoarthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/nanotherapeutic-approaches-for-the-treatment-of-post-traumatic-osteoarthritis/