Session Information
Date: Tuesday, November 19, 2024
Title: Abstracts: SLE – Diagnosis, Manifestations, & Outcomes III: Targets, Outcomes & Comorbidity
Session Type: Abstract Session
Session Time: 11:00AM-12:30PM
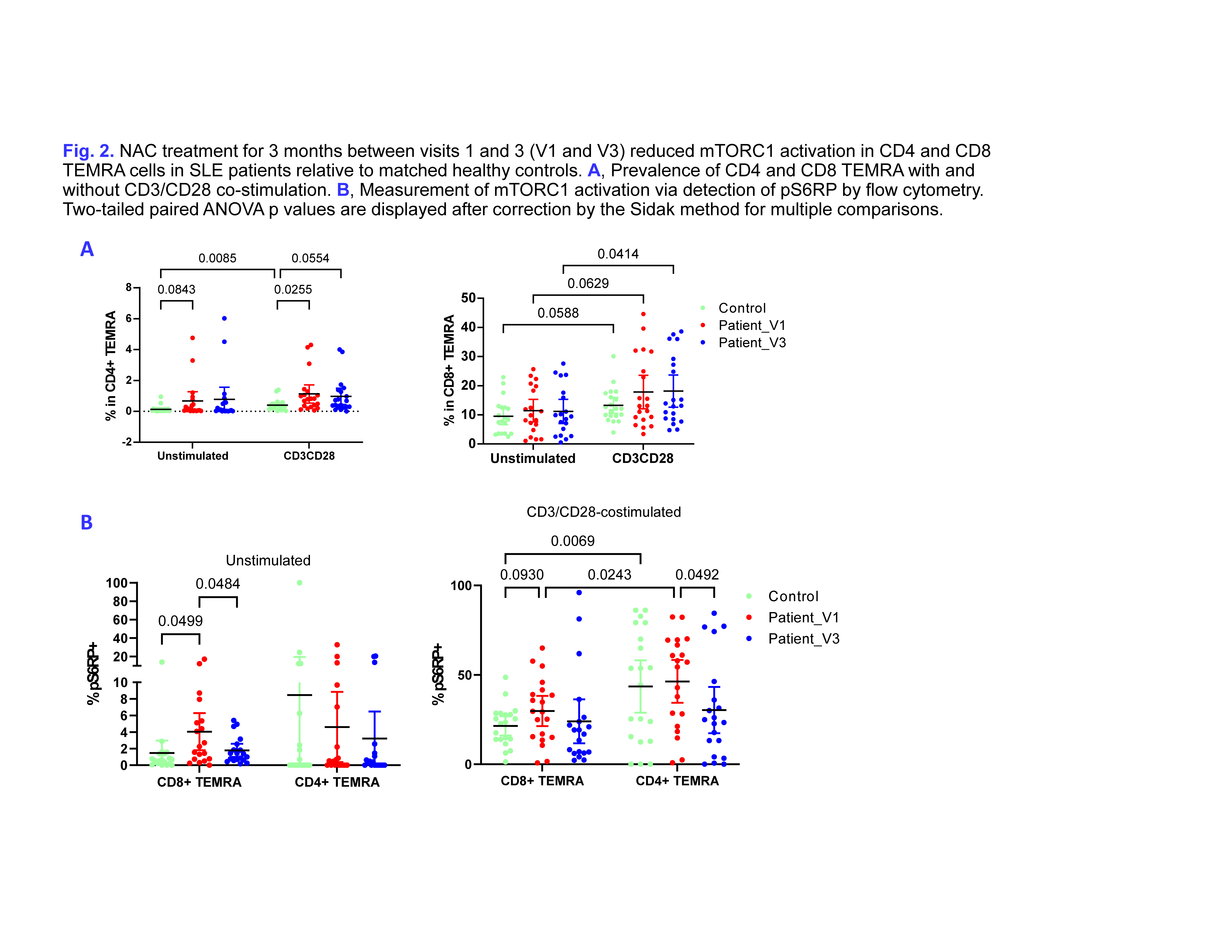
Background/Purpose: Systemic lupus erythematosus (SLE) is an autoimmune disease of unknown etiology with significant mortality attributed to infections due to toxicity of immunosuppressant medications. Our preliminary studies have shown that replacement of depleted glutathione with N-acetylcysteine (NAC) may reduce disease activity by blocking the mechanistic target of rapamycin (mTOR) in T cells of mice and patients with SLE (PMID: 22549432). Notably, mTOR-dependent expansion of cytotoxic CD8 effector-memory T cells re-expressing CD45RA (TEMRA) is considered pathogenic in SLE (PMID: 36893588). Therefore, we examined the impact of NAC on CD8 and CD4 TEMRA in the setting of a controlled clinical trial (clinicaltrials.gov NCT00775476).
Methods: Eligibility criteria required clinically active disease with ≥6 SLE Disease Activity Index (SLEDAI) and British Isles Lupus Assessment Group (BILAG) organ domain scores ≥1 BILAG A (A=12)or ≥2 BILAG B (B=8). All participants received NAC for 3 months, titrated up to a maximally tolerated dosage between 2.4 g/day and 4.8 g/day. Our preliminary analysis evaluated the immunobiological effects of NAC in 18 patients over the 3-month open-label phase. Human T cells in peripheral blood were analyzed for the expression of mTORC1 and mTORC2, in naive T cells (CD45RA+CD45RO-CD62L+CCR7+), central memory T cells (CM, CD45RA-CD45RO+CD62L+), effector memory T cells (TEM, CD45RA-CD45RO+CD62L-) and effector memory T cells re-expressing CD45RA (TEMRA, CD45RA+CD45RO-CD62L-CCR7-), as earlier (PMID: 29551338). Each patient was processed and compared to healthy subjects matched for gender, ethnicity, and age within 10 years, before and after a 3-month open-label dose titration phase. Statistical analysis was performed with two-tailed paired ANOVA using Sidak correction for multiple comparisons in GraphPad software.
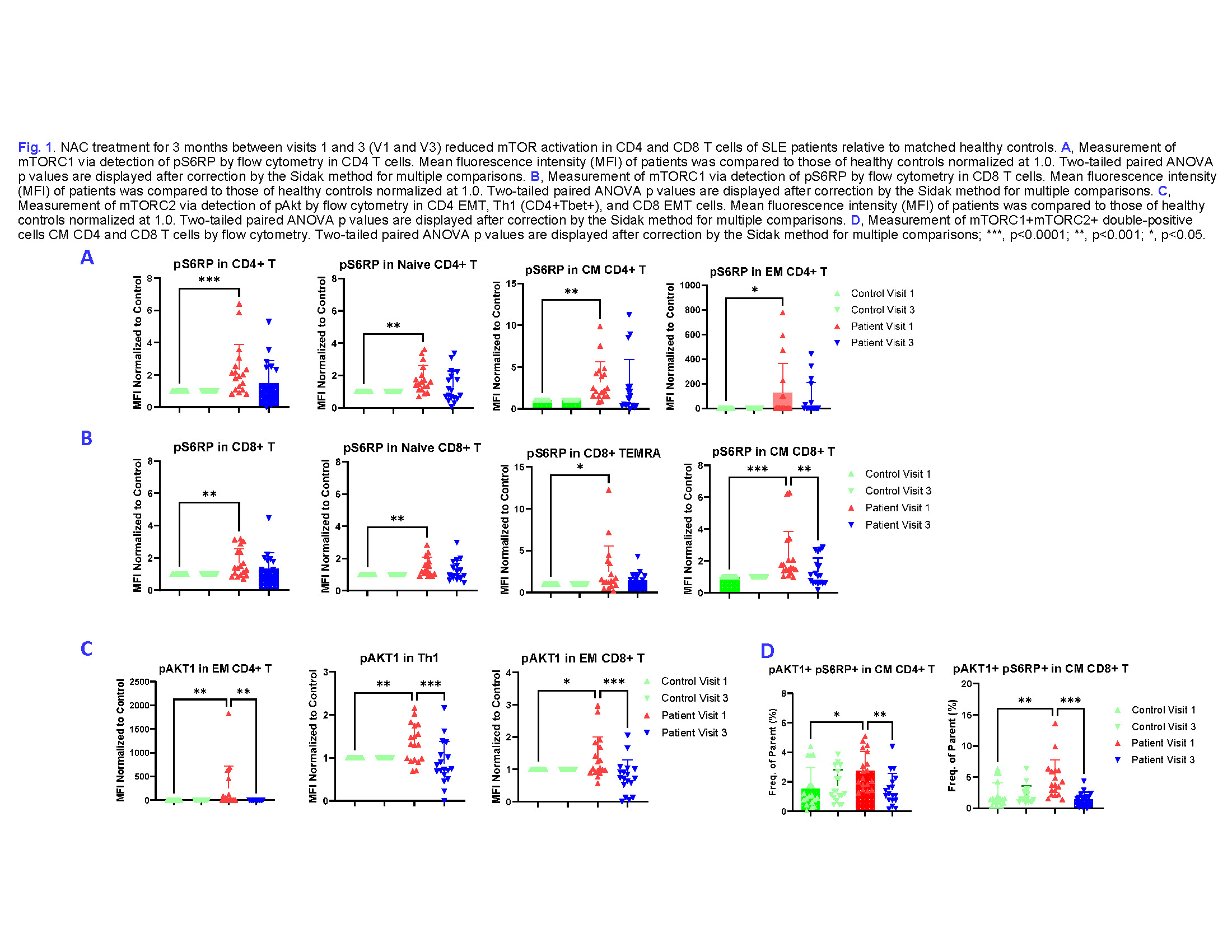
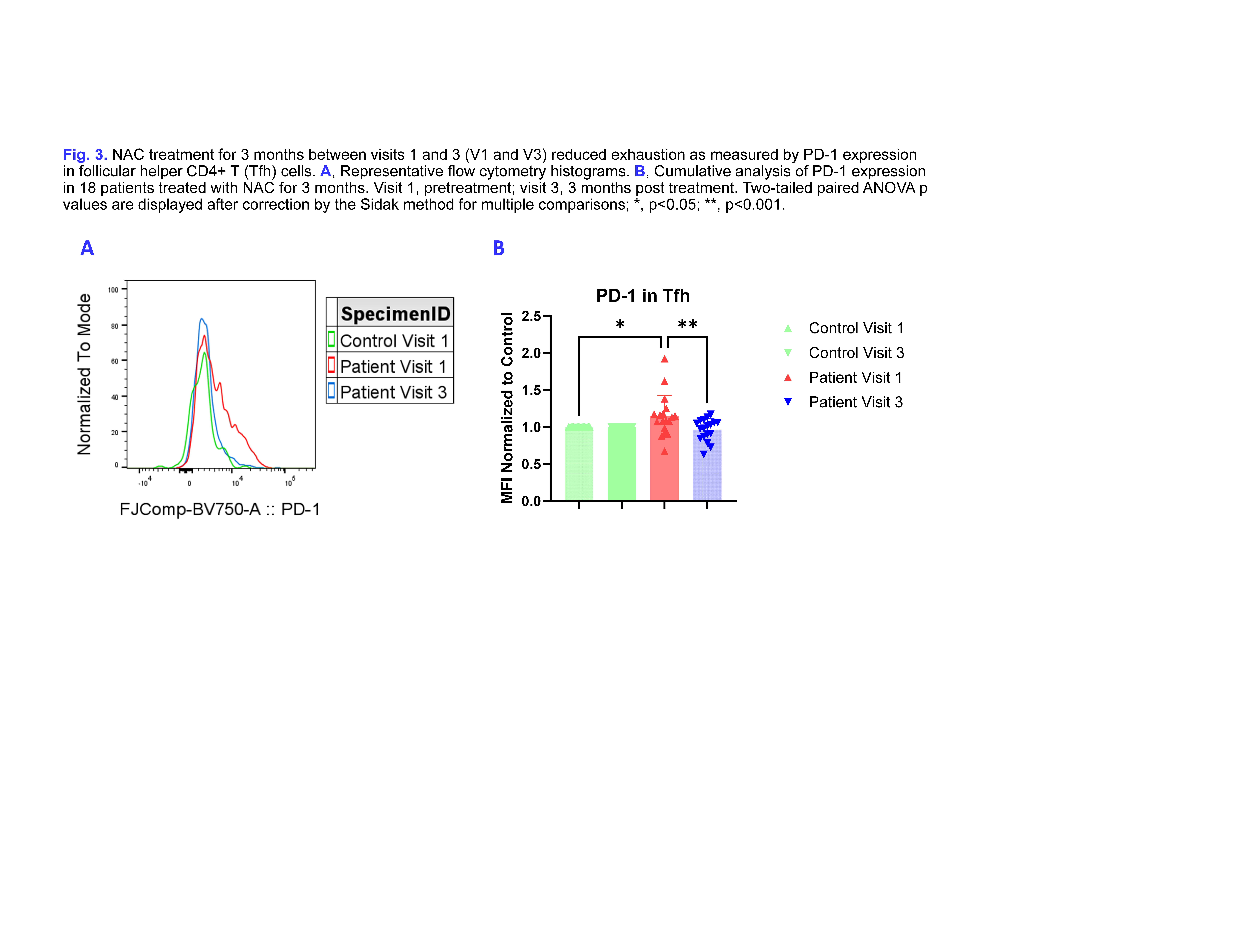
Results: We observed significant reduction of SLE disease activity as measured by diminished SLEDAI (-3.7±2.8; p=1.4×10-8) and BILAG in the 3-month open-label phase (-15.1±8.3; p=1.9×10-12). Flow cytometry of 18 analyzed SLE patients showed elevated mTORC1 (pS6RP) levels in both CD4+ T cells and CD8+ T cells, specifically in naïve (CD45RA+ CD45RO– CD62L+ CCR7+), central memory (CM, CD45RA– CD45RO+ CD62L+ CCR7+), and effector memory (EM, CD45RA– CD45RO+ CD62L– CCR7+) CD4+ T (Fig. 1A) as well as in naïve, CM, and TEMRA (CD45RA+ CD45RO– CD62L– CCR7–) CD8+ T cells (Fig. 1B). Post-treatment, elevated mTORC2 (pAKT1) in EM CD4+ T cells, EM CD8+ T cells, and Th1 (Tbet+GATA3–) CD4+ T cells (Fig. 2C) as well as elevated mTORC1/mTORC2 (pAKT1+ pS6RP+ cells) in CM CD4+ T cells and CM CD8+ T cells were also normalized by NAC treatment (Fig. 2D). Inreased pre-treatment exhaustion of follicular helper T cells (Tfh, CD4+ Bcl-6+) was also corrected after NAC treatment (Fig. 3).
Conclusion: This preliminary analysis supports the notion that NAC may serve as a safe and effective treatment via blocking mTOR in SLE patients. Both mTORC1 and mTORC2 activation were responsive to NAC treatment. Furthermore, NAC reversed the exhausted phenotype of Tfh cells in SLE patients, potentially contributing to its therapeutic benefits.
To cite this abstract in AMA style:
Park J, Ji L, Cabezas J, Wang X, Blaker B, Rao D, Godavarthy A, Blaker L, Ruchi F, Coman I, Olsen N, Lewis J, Ishimori M, Kirou K, Donath C, Kahlown S, Sereda D, Marte Furment M, Nasr S, Lokineni S, Ramsey-Goldman r, Weisman M, Weinstein A, Aranow C, Katalin B, McDermott M, Wallace D, Perl A. N-Acetylcysteine Blocks the Mechanistic Target of Rapamycin in Pro-Inflammatory Effector-Memory CD4 and CD8 T Cells Re-Expressing CD45RA in Patients with Active Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/n-acetylcysteine-blocks-the-mechanistic-target-of-rapamycin-in-pro-inflammatory-effector-memory-cd4-and-cd8-t-cells-re-expressing-cd45ra-in-patients-with-active-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/n-acetylcysteine-blocks-the-mechanistic-target-of-rapamycin-in-pro-inflammatory-effector-memory-cd4-and-cd8-t-cells-re-expressing-cd45ra-in-patients-with-active-systemic-lupus-erythematosus/