Session Information
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: Myositis is an infrequent but serious complication associated with immune check point inhibitor (ICI) treatment. Many patients with ICI-induced myositis will develop concurrent myocarditis and myasthenia gravis-like symptoms (ICI triad). We describe the prevalence and clinical characteristics of ICI-induced myositis and ICI triad among veterans receiving ICI.
Methods: Veterans enrolled in VHA who received ICI treatments between 6/6/2011 and 2/14/2023 were identified in the Corporate Data Warehouse. This analysis also employed data from the VA Central Cancer Registry for cancer diagnoses and Death Ascertainment File for all-cause mortality. Patients considered at high risk for ICI-induced myositis had medical records review via the Compensation and Pension Record Interchange (CAPRI) electronic medical records. Patients were considered at high risk if they met at least one of the following criteria: a creatine kinase (CK) value over 10-times the upper limit of normal (312 U/L) within six months of first ICI, the term “myositis” in a discharge summary any time after first ICI and/or term “myositis” in a progress note within six months after first ICI. ICI-induced myositis was defined by the presence of a provider diagnosis of ICI-induced myositis in CAPRI and laboratory or imaging evidence of myositis in the absence of alternate etiologies such as STEMI or septic shock. Mortality follow-up data was calculated in months from ICI initiation. Univariate testing by the Bartlett’s test and multivariate analysis by Cox regressions performed in Stata.
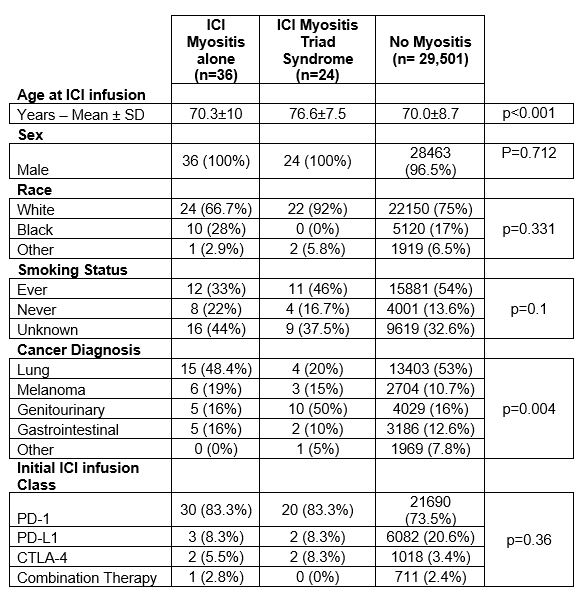
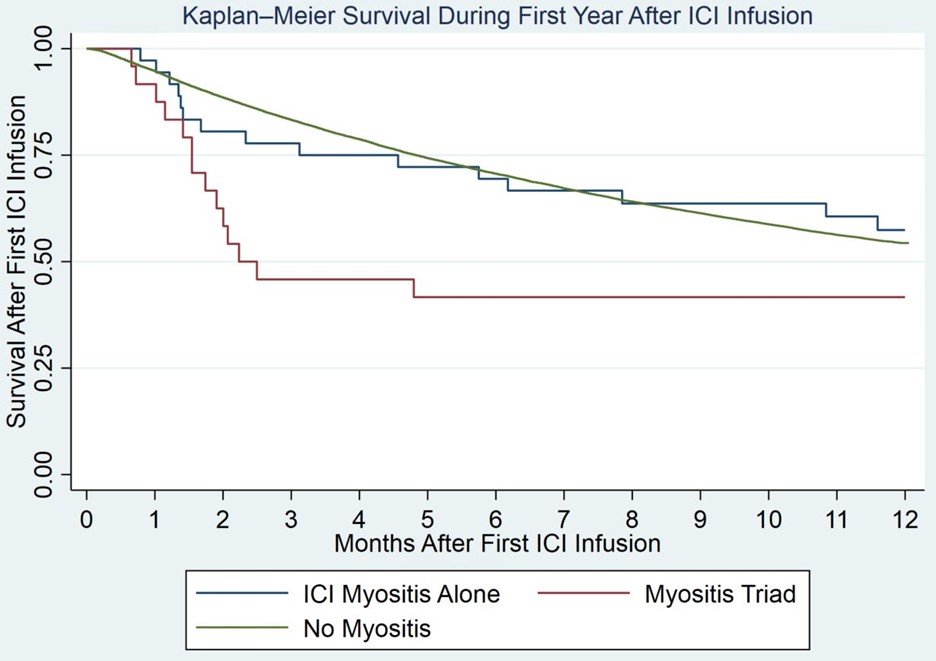
Results: We identified 60 patients with ICI-induced myositis of which 24 (40%) had the ICI triad out of 29,561 treated with ICIs. All patients with ICI-induced myositis were male. Self-defined race, ethnicity, and smoking status were similar between veterans treated with ICI who did not develop myositis, those who developed myositis alone, or ICI triad. ICI triad patients were significantly older compared to myositis alone patients (76.6±7.5 vs 70±10 years, p< 0.001). While PD-1 use was higher in the ICI-induced myositis groups, this increase was not statistically significant (83.3% vs 73.5%, p=0.36) (Table 1).Genitourinary cancers were more common in ICI triad populations than myositis alone (50% vs 15.6%, p=0.002). ICI triad patients had increased mortality in the first year of treatment than myositis alone patients but this difference just outside range for statistically significant (HR 1.65 [0.77-3.5], p=0.077 by Cox). Myositis alone patients had no significant mortality differences in mortality compared to patients without myositis (HR 1.01 [0.61,1.68], p=0.967 by Cox (Figure 1). These results were not confounded by either age or genitourinary status.
Conclusion: The ICI triad is a severe subset of ICI-induced myositis which is associated with older age at initial ICI treatment. A signal for potential association with ICI triad subset was seen with genitourinary cancers that will require further investigation. A strong trend for increased early mortality in the ICI triad subset closely following ICI initiation was observed in comparison to patients with myositis alone and ICI-treated veterans without myositis.
To cite this abstract in AMA style:
Rubino S, Cannon G, Sauer B, Rojas Jr J, Stoddard G, Kunkel G, Walsh J, England B, Mikuls T, Baker J, Braaten T. Myositis Triad Subset Is Associated with High Mortality in Immune Checkpoint Inhibitor- Induced Myositis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/myositis-triad-subset-is-associated-with-high-mortality-in-immune-checkpoint-inhibitor-induced-myositis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/myositis-triad-subset-is-associated-with-high-mortality-in-immune-checkpoint-inhibitor-induced-myositis/