Session Information
Session Type: ACR Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Dermatomyositis (DM) is an autoimmune disease affecting the skin, skeletal muscle, lungs, and/or other organs. While the pathogenesis remains poorly understood, it is thought to be driven largely by type 1 interferons (IFN-β) and involve CD4+ cells and dendritic cells. Plasmacytoid dendritic cells (pDCs) are considered major producers of type 1 interferons. Our objectives were as follows: 1) to quantify inflammatory cells and Type 1 IFN expression in skin and correlate with the Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI) skin activity score, 2) to identify cells contributing to refractoriness to hydroxychloroquine (HCQ), 3) to identify the major dendritic cell type producing IFN-β in DM.
Methods: In Aim 1, we evaluated lesional skin biopsies from 12 patients with moderate-severe cutaneous DM at baseline and from a nearby skin site after 12 weeks of treatment with lenabasum or placebo added to stable standard of care. Immunohistochemistry (IHC) was performed to assess expression of Type 1 IFN and various cell types [CD4+, macrophages, mDC (CD11c+), tissue-resident memory T (TRM) cells (CD69+), CD8+, mast cells, and pDC (CD123+)] and relationship with CDASI activity scores. RT-PCR was performed to measure Type 1 IFN gene expression. In Aim 2, HCQ responders and HCQ nonresponders were identified from a longitudinal DM database where responsiveness to HCQ was defined as sufficient improvement in skin lesions so that further escalation of therapy was not needed. IHC was performed on lesional skin biopsies to compare mDC and pDC expression. In Aim 3, flow cytometry was performed on PBMCs from 5 healthy controls and 5 DM patients to identify whether mDCs or pDCs were predominant producers of IFN-β in the two study populations. PBMCs were stimulated with Resiquimod (R848). Analysis was performed using FlowJo software. Statistical analysis included the Spearman rank correlation test and the Mann-Whitney test.
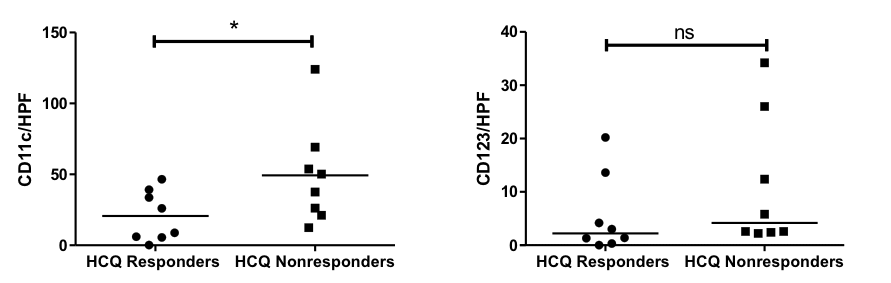
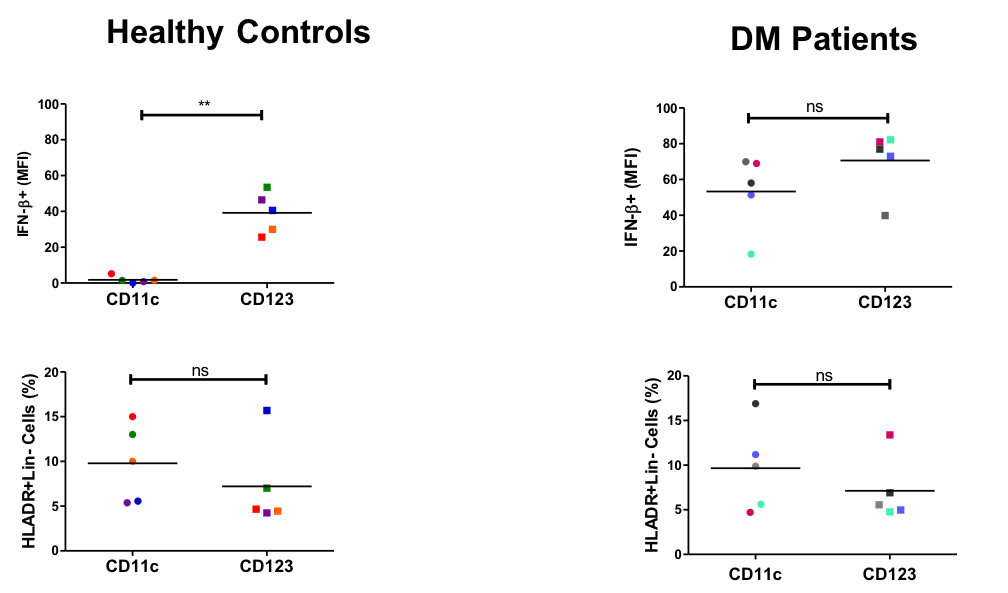
Results: In Aim 1, we found CD4+, macrophages, mDCs, and TRM cells were the most populous cells in DM lesional skin, followed by CD8+ cells, mast cells, and pDCs. Change in CD4+ cells/HPF, CD8+ cells/HPF, and mDCs/HPF significantly correlated with change in CDASI scores (r = 0.818, p = 0.001; r = 0.811, p = 0.001; r = 0.636, p = 0.026, respectively) (Fig. 1). Change in Type 1 IFN gene expression correlated with change in CDASI score (r = 0.559, p = 0.059) as did change in IFN-β protein expression (r = 0.629, p = 0.028) (Fig. 1). In Aim 2, significantly increased numbers of mDCs/HPF were found in skin of HCQ nonresponders (p < 0.05) (Fig. 2). In Aim 3, we found both mDCs and pDCs were major producers of IFN-β in DM patients; there was no significant difference in IFN-β production between the 2 cell types (Fig. 3). In contrast, in PBMCs from healthy controls, pDCs produced significantly more IFN-β compared to mDCs (p < 0.01) (Fig. 3). There was no significant different in percentage of mDCs vs. pDCs in healthy controls or DM patients (Fig. 3).
Conclusion: mDCs appear to play a significant role in DM pathogenesis. mDCs are major producers of IFN-β in DM patients but not in healthy controls. Increased numbers of mDCs are found in HCQ nonresponders.
To cite this abstract in AMA style:
Chen K, Zeidi M, Wysocka M, Reddy N, Jadoo A, Bashir M, Ahmed S, Patel B, Zhang K, White B, Werth V. Myeloid Dendritic Cells (mDCs) Are Major Producers of Interferon-β in Dermatomyositis and Higher Numbers of mDCs Are Found in Hydroxychloroquine Nonresponders [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/myeloid-dendritic-cells-mdcs-are-major-producers-of-interferon-%ce%b2-in-dermatomyositis-and-higher-numbers-of-mdcs-are-found-in-hydroxychloroquine-nonresponders/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/myeloid-dendritic-cells-mdcs-are-major-producers-of-interferon-%ce%b2-in-dermatomyositis-and-higher-numbers-of-mdcs-are-found-in-hydroxychloroquine-nonresponders/