Session Information
Date: Saturday, November 12, 2022
Title: Abstracts: Systemic Sclerosis and Related Disorders – Clinical I: Trials and Therapeutics
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: In the randomized Scleroderma: Cyclophosphamide Or Transplantation (SCOT trial), normalization of systemic sclerosis (SSc) peripheral blood gene expression signatures at the 26-month visit was observed after myeloablative hematopoietic stem cell transplantation (HSCT) (Assassi, et al., 2019). Herein, we examined long-term molecular changes ensuing after HSCT or 12 months of intravenous cyclophosphamide (CYC) to 54 months after randomization.
Methods: All participants with available peripheral blood total RNA samples at month 38 or month 54 visits were included in the present study. Sixty-two healthy controls of similar age-, gender-, and racial background were also examined. Global transcriptomic studies were performed at pretreatment baseline, 38 months, and 54 months post-randomization, and in healthy controls using Illumina HT-12 arrays. The association of baseline SSc-related transcript module scores (upregulated modules: two interferon modules and neutrophil module; down-regulated module: cytotoxic/NK module) with longitudinal changes in modified Rodnan Skin Score (mRSS) was also examined in each treatment arm using mixed effects regression models (Keyes-Elstein et al., 2021).
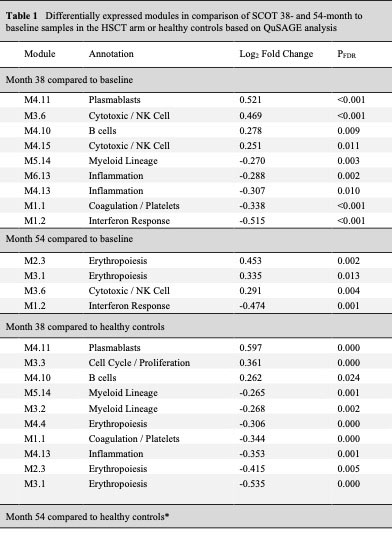
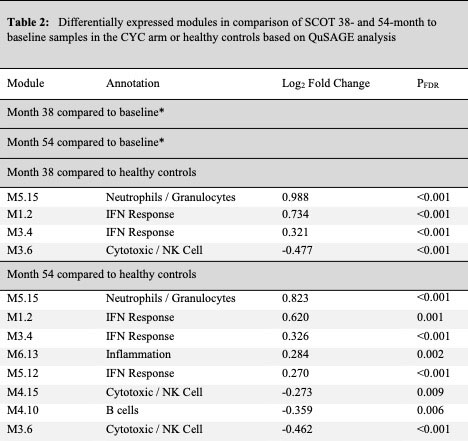
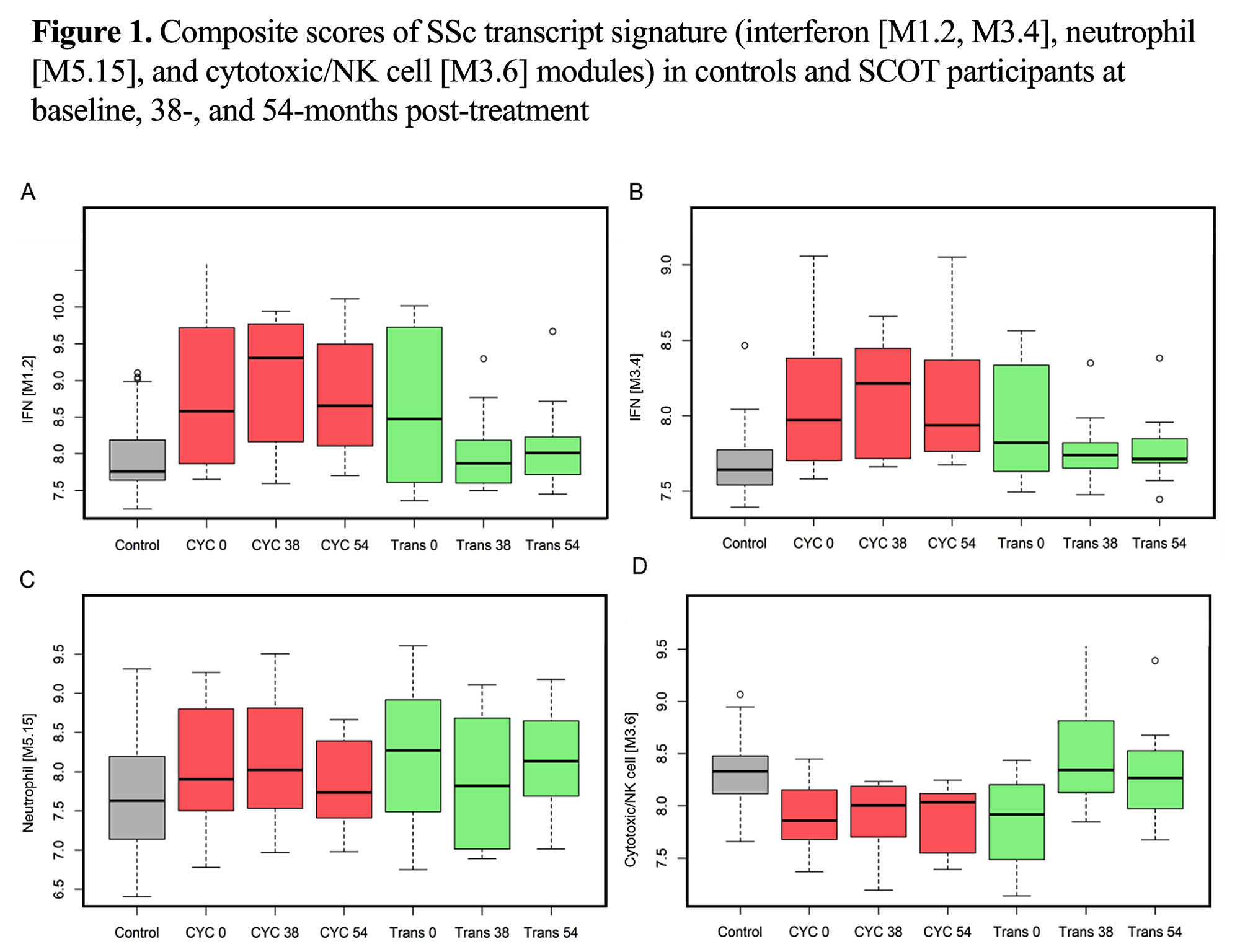
Results: Thirty (HSCT=19 and CYC=11) participants had 38-month and 26 (HSCT=16 and CYC=11) had 54-month samples available. In the paired comparison to the baseline visit, a significant down-regulation of interferon and an up-regulation of cytotoxic/NK module were observed at 38 and 54-months in the HSCT arm, indicating a long-term normalization of baseline SSc gene expression signatures (Table 1/Figure 1). No differentially expressed modules were detected in the CYC arm compared to baseline (Table 2).
In comparison to healthy controls, 38-month visit samples in the HSCT showed an upregulation of B cell and plasmablast modules and a downregulation of myeloid and inflammation modules. Importantly, 54-month transplant samples showed no differentially expressed modules compared to healthy controls suggesting completion of immune reconstitution (Table 1/Figure 1). Participants in the CYC arm continued to show an upregulation of interferon and neutrophil modules and a down-regulation of cytotoxic/NK module compared to healthy controls at both time points (Table 2).
Higher baseline interferon transcript modules were associated with a faster rate of decline in mRSS in the HSCT arm (p=0.015 and p=0.005 for M1.2 and M3.4 interferon modules, respectively) but not in the CYC arm (p=0.132 and p=0.202 for M1.2 and M3.4 interferon modules, respectively).
Conclusion: Paralleling the observed clinical benefit, HSCT leads to durable long-term normalization of molecular signature in SSc with completion of immune resetting to 54 months post-HSCT.
To cite this abstract in AMA style:
Wareing N, Wang X, Keyes-Elstein L, Goldmuntz E, Lyons M, McSweeney P, Furst D, Nash R, Crofford L, Welch B, Pinckney A, Mayes M, Sullivan K, Assassi S. Myeloablation Followed by Hematopoietic Stem Cell Transplantation Leads to Long-term Normalization of Systemic Sclerosis Molecular Signatures [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/myeloablation-followed-by-hematopoietic-stem-cell-transplantation-leads-to-long-term-normalization-of-systemic-sclerosis-molecular-signatures/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/myeloablation-followed-by-hematopoietic-stem-cell-transplantation-leads-to-long-term-normalization-of-systemic-sclerosis-molecular-signatures/