Session Information
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Treatment of dermatomyositis (DM) typically follows a stepwise sequence starting with either methotrexate (MTX) or mycophenolate mofetil (MMF) after an inadequate response to antimalarial therapy. However, data is scarce regarding the effectiveness of MTX and MMF.
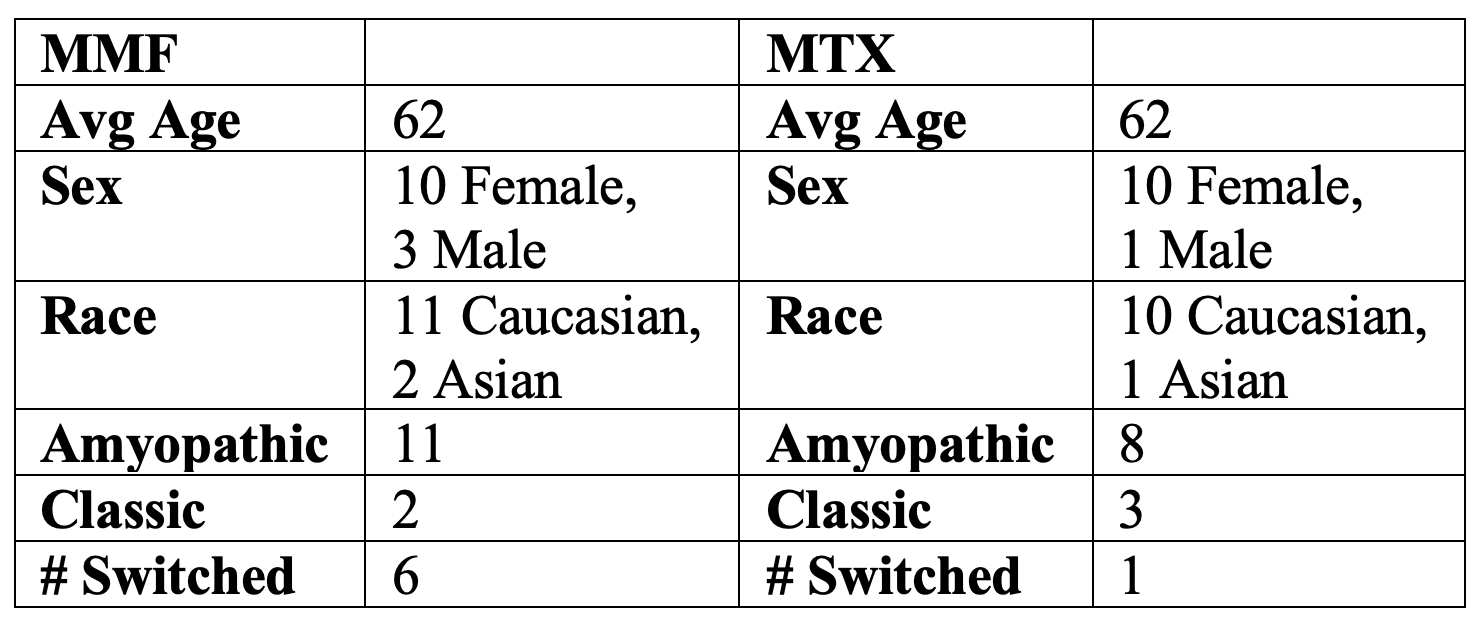
Methods: A cohort of 24 patients with currently skin-predominant DM was identified using data from a prospective database at The University of Pennsylvania. Included patients took MTX or MMF and had ≥ two study visits within a retrospective observation period from October 2008 until February 2021. The Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI) was used to assess severity and outcomes. Patients with mild disease activity, defined as a CDASI activity (CDASI-A) score < 14 (maximum sub-score of 100), were excluded from the analysis as were any patients on any other medications used to treat DM aside from MTX or MMF, with the exception of chronic antimalarials or topical medications.
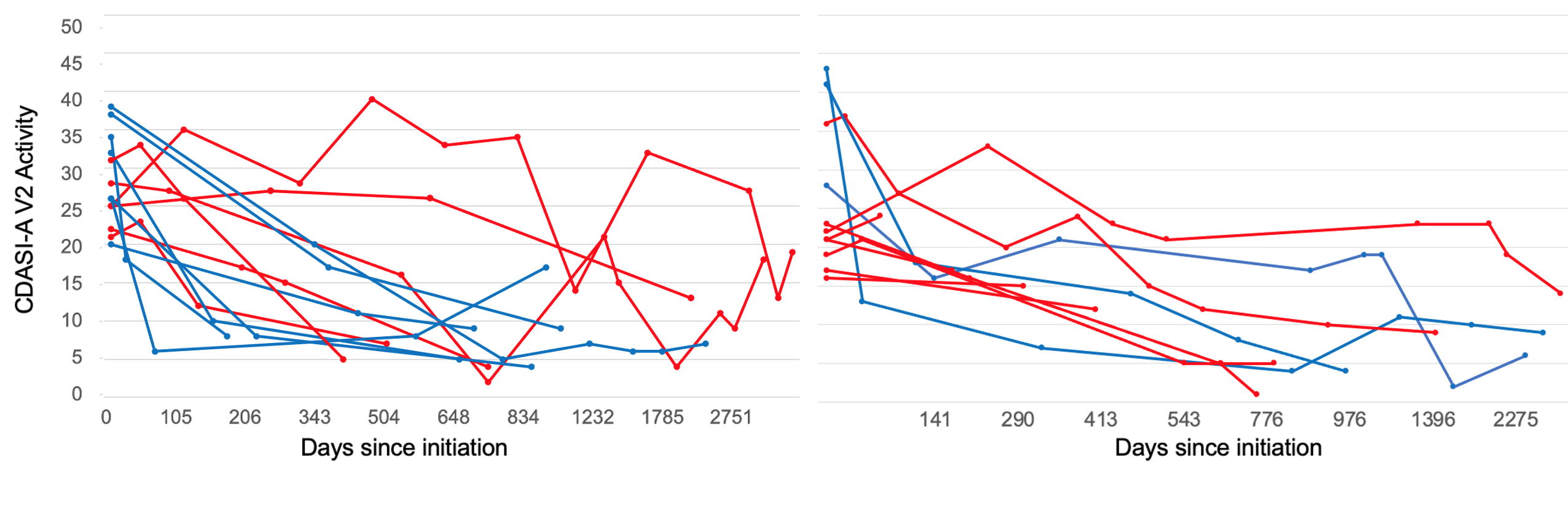
Results: For both MMF (n=13) and MTX (n=11), there was no baseline difference in CDASI-A scores at initiation. There was no significant difference in the degree of improvement on either medication, with a mean difference in daily CDASI-A change between MTX and MMF of -0.0028 ± 0.0024 (p=0.2400) using a mixed linear effects model. For MTX, the median percentage change in CDASI-A between the first and last study visit was -74%. For MMF, the median percentage change was -76%. A decrease of 40% or greater in the CDASI-A score has previously been associated with a meaningful change in quality of life (1). Defining responders as having a 40% or greater improvement in their CDASI-A score between their first and second observations (see Figure 1), 27% of the patients taking MTX were responders while 54% of patients taking MMF were responders. The range of time varied between the first and second visits with 50% of patients having a second study visit within 150 days. By last follow-up, 55% of patients taking MTX were considered responders and 77% of patients taking MMF met criteria to be considered responders. For MTX, the median follow-up for the second visit was 178 days and for the last visit was 776 days. For MMF, the median follow up was similar—147 days for the second visit, and for the last visit was 787 days. Of note, 7 patients in total had taken both MMF and MTX at some point in their disease course. More patients switched from taking MTX to MMF (see Table 1).
Conclusion: Although we do not have a large enough sample size to make a properly powered conclusion, MTX trended to a slightly delayed effect compared to MMF. Either MMF or MTX may be added to treatment plans for patients with DM who have not responded to antimalarial therapy. Moreover, our data suggest that responders continued to improve over many months (even years for some) while most non-responders showed little improvement at first follow-up (ranging from 2-6 months) during the observation period. Large cross over studies with safety data are needed to evaluate patient response to MMF and MTX in DM.
References
1. Ahmed S, Chakka S, Concha J, Krain R, Feng R, Werth VP. Evaluating important change in cutaneous disease activity as an efficacy measure for clinical trials in dermatomyositis. Br J Dermatol. 2020;182(4):949-54.
(*Responders at first follow up displayed in blue, nonresponders displayed in red)
To cite this abstract in AMA style:
Grinnell M, Keyes E, Diaz D, Vazquez T, Feng R, Werth V. Mycophenolate Mofetil and Methotrexate Efficacy in Dermatomyositis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/mycophenolate-mofetil-and-methotrexate-efficacy-in-dermatomyositis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mycophenolate-mofetil-and-methotrexate-efficacy-in-dermatomyositis/