Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: The overall management of SLE and Lupus Nephritis (LN) has significantly improved over the last four decades.Recently great therapeutic strides have been made in management of LN using newer agents alone or combination of older therapies. With the availability of increased number of options choosing the optimal treatment regimen for LN has become challenging. Identifying the treatment regimen most effective in achieving complete response while minimizing adverse events is crucial for improving patient outcomes.We performed a systematic review and meta-analysis of clinical trials to explore optimal treatment regimen for LN.
Methods: The systematic review and meta-analysis were performed using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The literature search was conducted using public databases, such as the Cochrane Library, Web of Science and PubMed. Only randomized controlled clinical trials with either active comparator or placebo arms were included. Studies were excluded if they were retrospective, with no comparative drug arm, or less than 20 subjects per treatment response arm. Due to lack of agreed upon outcome measures, complete response (CR) and partial response (PR) criteria were determined as per the definition used by individual studies. Severe adverse reaction (SAE) frequencies were determined for each study for safety evaluation. Data from each study were evaluated for consistency. Publication bias was detected by using funnel plots. Forest plots were used to summarize data, generate frequencies, odds ratio (OR), relative risks (RR) and corresponding 95% confidence intervals (CI) for each subgroup. Heterogeneity was evaluated using the I2statistic, and labelled as low (25%), moderate (50%) and high (75%). All analyses were performed using R Statistical Software meta package (v4.2.1; R Core Team 2022).
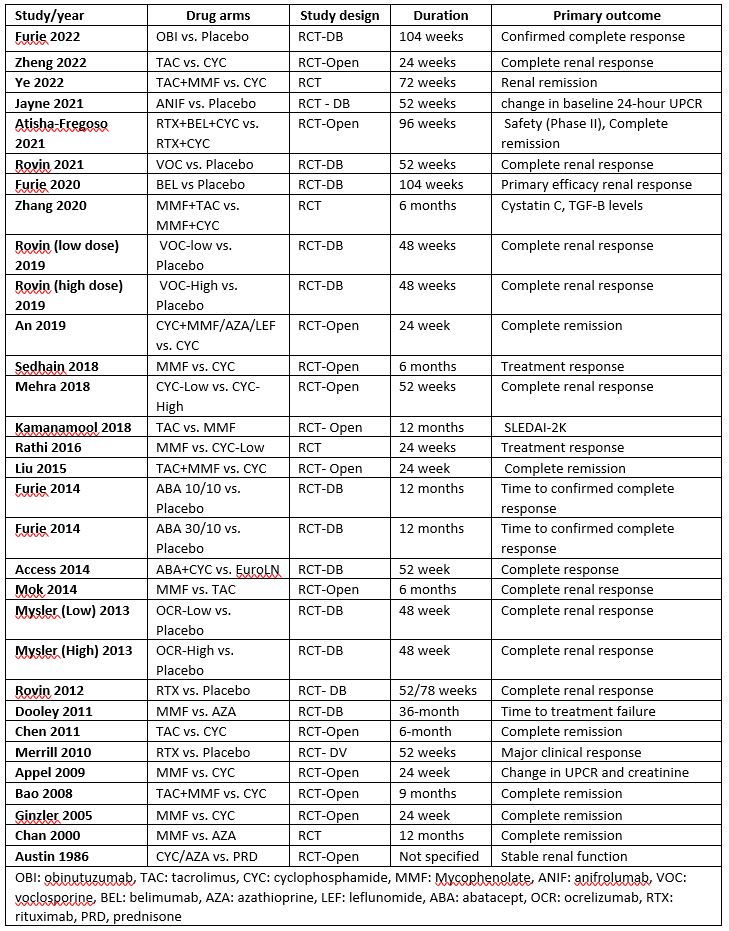
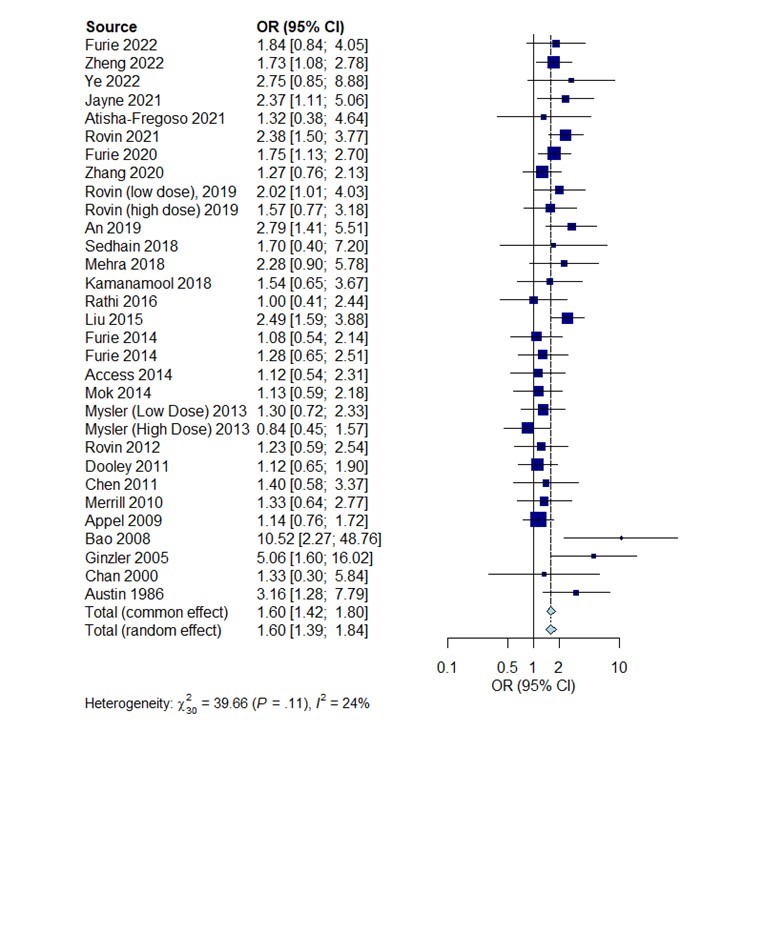
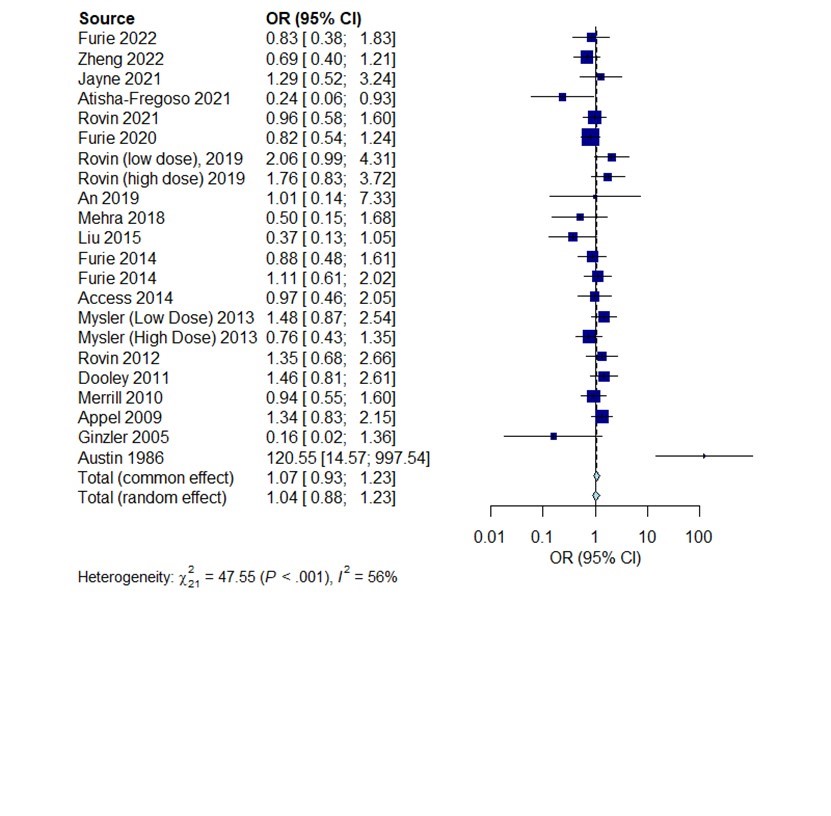
Results: 31 eligible studies comprising 5495 SLE patients were included in analyses, the study characteristic features are shown in Table 1. Overall significant OR for achieving CR was reported by 10 studies (Figure 1). The highest effect was reported from study by Bao et.al using a combination of tacrolimus(TAC) and mycophenolate mofetil (MMF) compared to cyclophosphamide (CYC) (OR=10.5, 95% CI 2.27; 48.76), another study using TAC+MMF combo reported OR=2.49, 95% CI 1.59;3.88. The study by Ginzler et.al reported OR= 5.06 (95% CI 1.6:16.02) in favor of using MMF. Studies using CYC, voclosporin + MMF, and belimumab also reported significant OR for achieving CR. Data for the frequency of SAE were available in 22 studies. The frequency of SAE was not significantly different between treatments arms (OR: 1.07, 95%CI: 0.93-1.23, p >0.05)
Conclusion: Our meta-analysis suggests multi-target treatment strategies, by adding calcineurin inhibitors to MMF may be better for achieving complete response in LN. Furthermore, using this combination treatment did not seem to increase the risk of side effects. Heterogenous treatment response criteria and variable comparator arms were major limiting factors of this study.
To cite this abstract in AMA style:
Pamuk O, Shaikh B, Khan S, Abbas A, Abi Doumeth S, Hasni s. Multi-targeted Therapy Better in Achieving Complete Response in Lupus Nephritis: A Systematic Review and Meta-analysis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/multi-targeted-therapy-better-in-achieving-complete-response-in-lupus-nephritis-a-systematic-review-and-meta-analysis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/multi-targeted-therapy-better-in-achieving-complete-response-in-lupus-nephritis-a-systematic-review-and-meta-analysis/