Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The clinical complications and symptoms of systemic lupus erythematosus (SLE) can vary widely with the individual and appropriate management is critically dependent upon the proper assessment of disease activity and organ damage. Although antibodies such as anti-dsDNA have been shown to correlate with disease activity and the likelihood of nephritis in lupus patients, the additive effect of combined biomarkers is not widely implemented. Using a cohort of well characterized SLE patients, this study investigated the utility of developing an SLE assessment model using a combination of biomarkers, namely anti-dsDNA, anti-C1q, and anti-Ku antibodies.
Methods: Samples from 261 SLE patients from Linköping University (Sweden) were tested using chemiluminescent immunoassays (CIA) for anti-dsDNA and anti-Ku (research use only) antibodies as well as by anti-C1q ELISA (all methods Inova Diagnostics, San Diego, USA). Of these 261 SLE patients, 69 (26.4%) had lupus nephritis (LN) at the time of the blood draw, or had the history of LN. The SLE disease activity index-2K (SLEDAI) scores were available for all patients and a cut-off >4 was used to define active disease (50/261, 19.2% active). The data were statistically evaluated using Analyse-it software (Version 3.90.1; Leeds, UK). The multi-parametric analysis was performed at both the manufacturer’s cut-off for the methods as well as optimized cut-off points based on likelihood plots to increase the odds ratio.
Results: In the total cohort, all three antibodies (dsDNA, C1q, and Ku) demonstrated markedly higher prevalence and quantitatively higher antibody levels in active SLE patients versus inactive patients and in LN patient versus non-LN patients (see table).
|
Anti-dsDNA |
Anti-C1q |
Anti-Ku |
||||
|
Antibody titer level |
Antibody prevalence |
Antibody titer level |
Antibody prevalence |
Antibody titer level |
Antibody prevalence |
|
| Active vs. Inactive |
p<0.0001 |
p<0.0001 Odds ratio = 4.2 |
p<0.0001 |
p<0.0001 Odds ratio = 6.9 |
p=0.0012 |
p=0.3343 Odds ratio = 1.8 |
| LN vs. non-LN |
p<0.0001 |
p=0.0002 Odds ratio = 2.9 |
p<0.0001 |
p<0.0001 Odds ratio = 4.4 |
p=0.0213 |
p=0.1625 Odds ratio = 2.1 |
When multi-parametric analysis was performed by combining biomarker results, the likelihood of nephritis and patients with active disease increased with dual positivity and triple positivity (see figure). 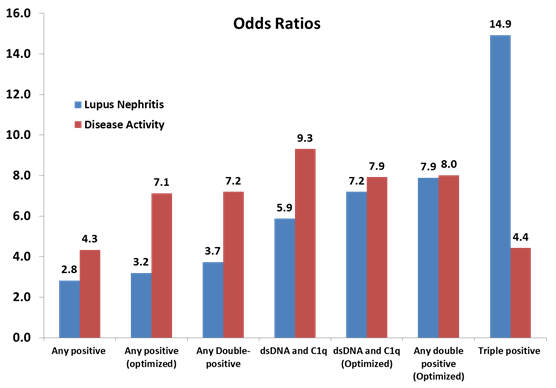
Conclusion: This study demonstrates the utility of a multi-parametric model approach using biomarkers for assessing SLE patients for more active and severe disease, especially for patients that may develop lupus nephritis. Furthermore, the results hold promise for the benefit of combined autoantibody profiling for the clinical management of patients.
To cite this abstract in AMA style:
Sjöwall C, Bentow C, Aure MA, Lakos G, Martis P, Mahler M. Multi-Parametric Model Development for Assessing Lupus Nephritis and Disease Activity [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/multi-parametric-model-development-for-assessing-lupus-nephritis-and-disease-activity/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/multi-parametric-model-development-for-assessing-lupus-nephritis-and-disease-activity/
