Session Information
Date: Saturday, November 12, 2022
Title: SLE – Diagnosis, Manifestations, and Outcomes Poster I: Diagnosis
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Systemic lupus erythematosus (SLE) is a complex, systemic autoimmune disease that interferes with the balance between regulation and immunity, resulting in immune system dysfunction. Disease course is unpredictable due to alternating remissions and flares, impeding reliable patient assessments. Thus, mechanistic insights are required for better assessment. Current studies are mainly descriptive, and SLE is best interrogated with a multi-parametric, holistic approach such as mass cytometry (CyTOF).
The objectives of this study are as follows: (1) To characterise immune signatures of SLE patients and in the process: Study the roles of B and T cells in SLE to gain a holistic understanding of the adaptive immune response and (2) To compare immunological profiles of SLE patients with age-matched healthy controls.
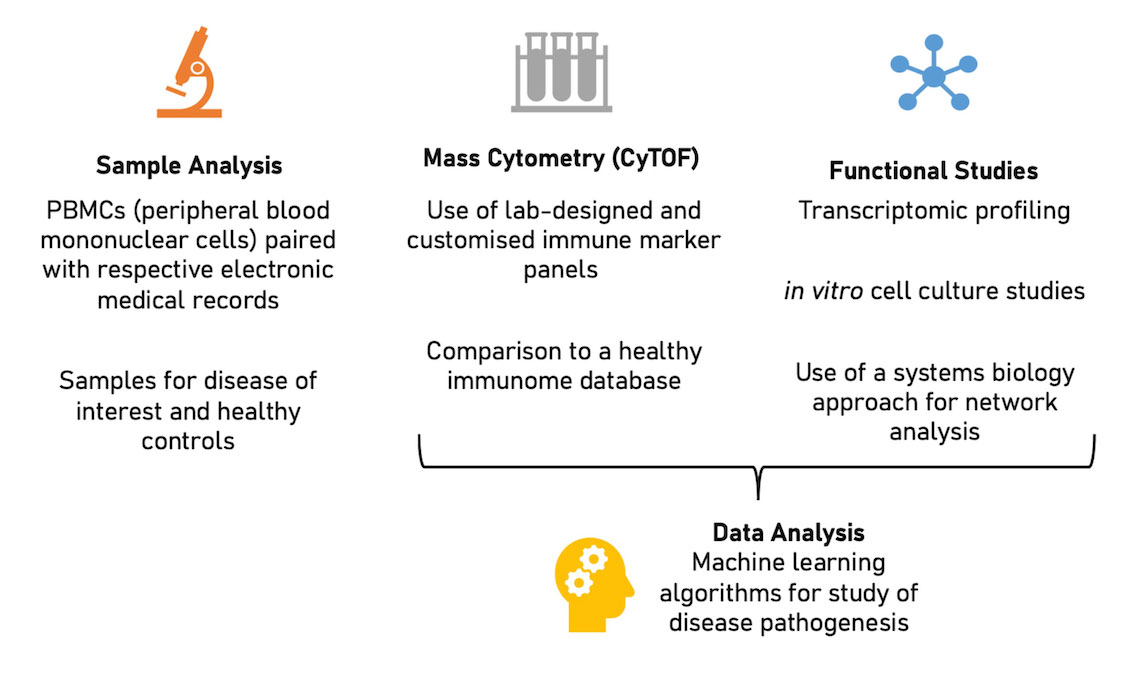
Methods: Peripheral blood mononuclear cells (PBMCs) from 26 SLE subjects (41 samples, median age 39.5years) and 27 age-matched subjects were tested with CyTOF. Data was analysed and visualised using our laboratory-designed machine learning tool, the Extended Polydimensional Immunome Characterization (EPIC) platform. Normalization and FlowSOM (Flow cytometry analysis by Self- Organising Maps) clustering were performed with functionally and phenotypically important immune markers. Mann-Whitney U test identified significantly different cluster frequencies.
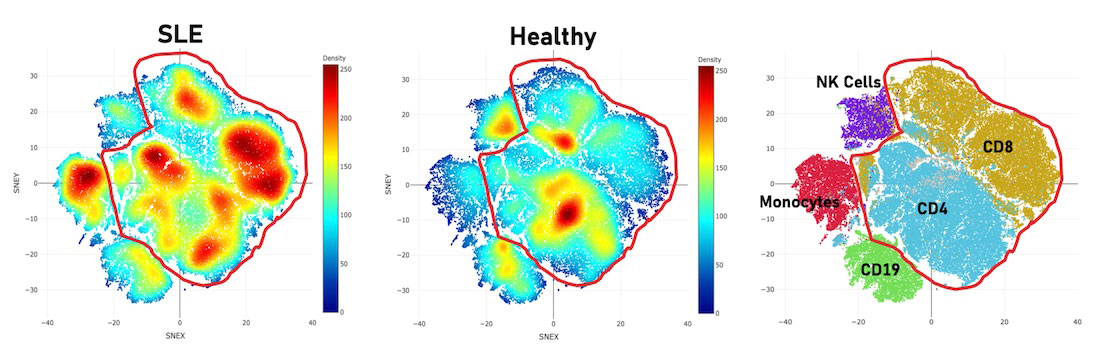
Results: Multiple significant differences were identified (p < 0.05). An activated CD8+CXCR3+CLA+ T -cell subset (CD8+CD45RA+CD38+CLA+CXCR3+) was enriched in SLE (median: 0.19%,interquartile range: 0.13-0.35% of CD45+ PBMCs) versus healthy (0.08%,0.05-0.09%; p< 0.0001). CXCR3 and cutaneous lymphocyte-associated antigen (CLA) are involved in skin-homing, with CLA function enhanced in inflammatory dermatoses. This suggests the importance of skin involvement in SLE immunopathogenesis even if skin manifestations are absent. Further analysis of CD3+ cells revealed unchanged natural T-regulatory cells (TREGS) in healthy and disease while a few TREG-like populations (FoxP3+CD25-) are significantly increased in disease, suggesting a deranged immunoregulatory response driven by TREGS. Thirdly, an activated CD8+BAFF+ T-cell subset (CD8+CD45RA+iCOS+ BAFF+) (r=0.715, p< 0.0001) is enriched in SLE (0.975%,0.433-1.53%) versus healthy (0.28%,0.1-0.49%; p< 0.0001). B-cell activating factor (BAFF) supports autoreactive B cell survival in autoimmune disease; an anti-BAFF drug (belimumab) is FDA- approved for SLE. Despite increasing use of belimumab, not all patients respond equally, so BAFF inhibition alone may not adequately alter disease activity. Studying these cell subset interactions in further detail to identify pathological pathways may facilitate improvements in current SLE theragnostics.
Conclusion: With a multi-parametric unbiased approach comparing SLE subjects to healthy controls, we identified immune subsets of immunopathogenic importance for further studies.
To cite this abstract in AMA style:
Nay Yaung K, Yeo J, Law H, Wasser M, Arkachaisri T, Thumboo J, Low A, Albani S. Multi-Parametric Interrogation of the Systemic Lupus Erythematosus (SLE) Immunome Reveals Multiple Derangements [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/multi-parametric-interrogation-of-the-systemic-lupus-erythematosus-sle-immunome-reveals-multiple-derangements/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/multi-parametric-interrogation-of-the-systemic-lupus-erythematosus-sle-immunome-reveals-multiple-derangements/