Session Information
Date: Monday, October 27, 2025
Title: (1088–1122) Immunological Complications of Medical Therapy Poster
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
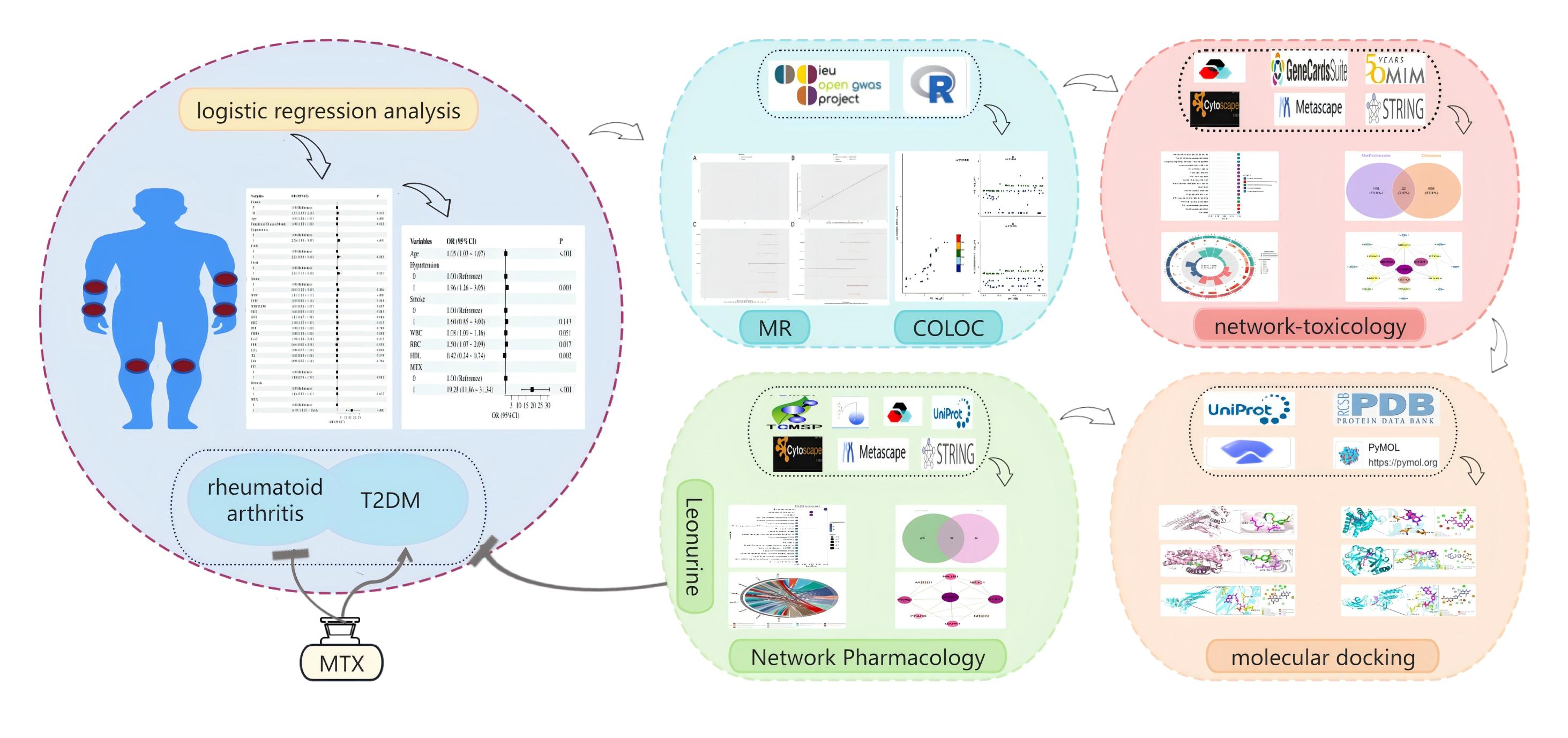
Background/Purpose: Methotrexate (MTX) is a first-line treatment for autoimmune diseases, including rheumatoid arthritis (RA). Its potential risk of inducing type 2 diabetes mellitus (T2DM) remains controversial, and the underlying mechanism is not well understood.
Methods: This study utilized a multi-omics integration approach to systematically assess the association mechanism between MTX and T2DM. Initially, a binary logistic regression model was constructed based on clinical cohort data from 1,253 RA patients, to analyze the correlation between MTX exposure and glycemic metabolism indicators. Additionally, genetic instrumental variables associated with MTX metabolism were selected using public Genome-Wide Association Study (GWAS) databases. The causal relationship was evaluated employing a two-sample Mendelian randomization (MR) framework, combined with inverse variance weighted (IVW), weighted median (WM1), MR-PRESSO, and MR-Egger regression methods, and Bayesian colocalization analysis was used to pinpoint shared genetic variations across the genome. At the molecular mechanism level, a network toxicology approach was utilized to construct an MTX-T2DM interaction network. Molecular docking validation was then performed on selected core targets to confirm their affinity. For potential intervention strategies, the network pharmacology approach was employed to predict the multi-target action network of leonurine, and molecular docking techniques were applied to evaluate the binding stability with key targets.
Results: Clinical data indicates a significant positive correlation between MTX exposure and the risk of T2DM (aOR=19.28, 95%CI:11.86-31.34). MR analysis reveals a significant positive correlation between MTX exposure and the risk of T2DM (OR: 4.50, 95% CI: 3.62-5.61, p=4.03E-31). Bayesian colocalization analysis demonstrates significant genomic colocalization between genetic variants associated with MTX drug response and T2DM risk loci, suggesting that both conditions share causal genetic drivers. Network toxicology and molecular docking elucidate that MTX disrupts fat differentiation and aggravates insulin resistance by inhibiting the PPARG ligand binding domain (docking energy -6.69 kcal/mol) and affecting lipid storage and metabolism. Notably, network pharmacology predictions, combined with molecular dynamics simulations, have confirmed that leonurine can target and activate PPARG and interfere with the Toll-like receptor signaling pathway, thereby reversing the metabolic-immune imbalance induced by MTX.
Conclusion: This study has, for the first time, constructed a four-dimensional interaction model of “drug-gene-metabolism-immunity” based on clinical data, proposed a multi-target cascading mechanism of MTX-induced T2DM, and provided interdisciplinary evidence chains for the use of leonurine as a precise intervention agent. This lays a theoretical foundation for optimizing clinical medication with MTX and strategies for the prevention and treatment of T2DM.
To cite this abstract in AMA style:
Shi M, Zou F, Ma X, Feng W, Zhang Y, Lin C, Liu M, Xu Q. Multi-omics Integration Analysis of the Cross-scale Mechanism of MTX-induced T2DM Risk and the Precise Intervention Strategy of Leonurine [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/multi-omics-integration-analysis-of-the-cross-scale-mechanism-of-mtx-induced-t2dm-risk-and-the-precise-intervention-strategy-of-leonurine/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/multi-omics-integration-analysis-of-the-cross-scale-mechanism-of-mtx-induced-t2dm-risk-and-the-precise-intervention-strategy-of-leonurine/