Session Information
Date: Monday, November 14, 2022
Title: Abstracts: T Cell Biology and Targets in Autoimmune and Inflammatory Disease
Session Type: Abstract Session
Session Time: 3:00PM-4:00PM
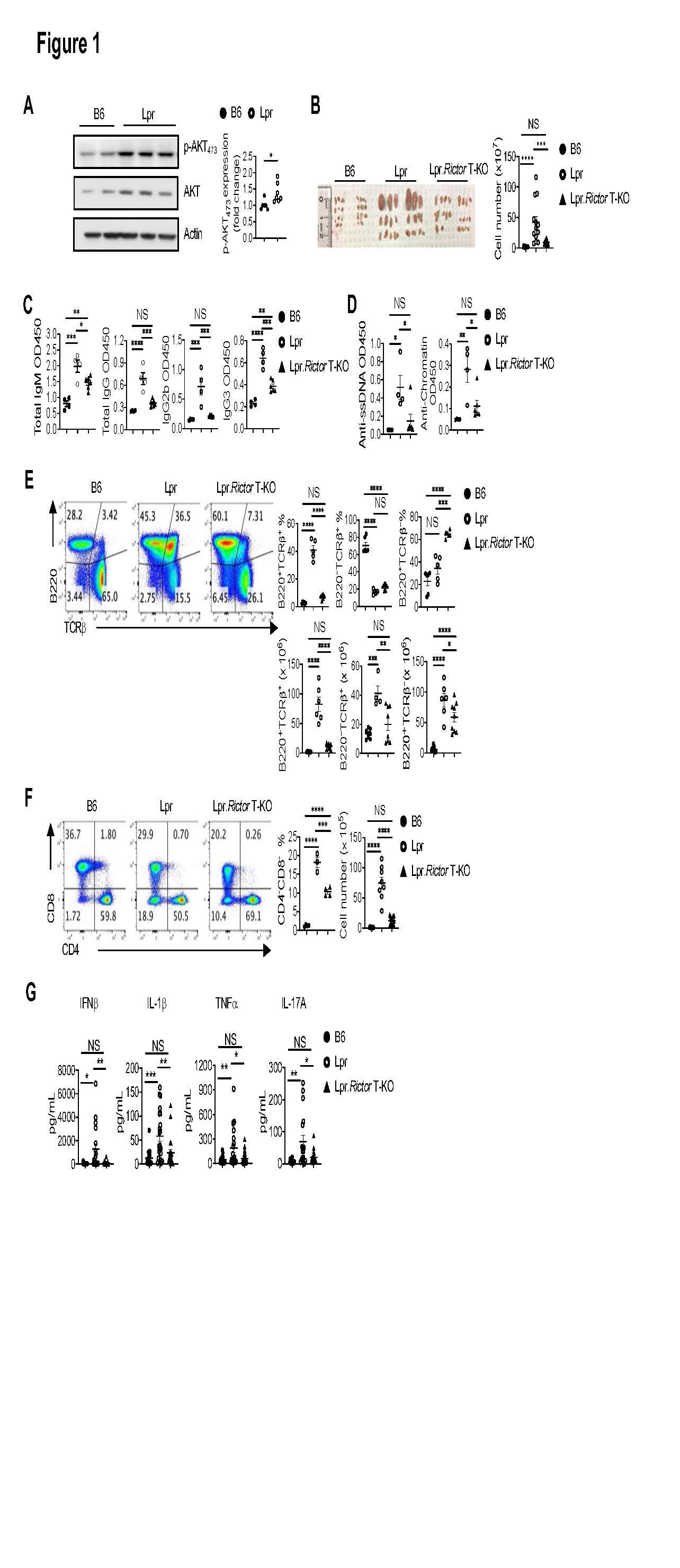
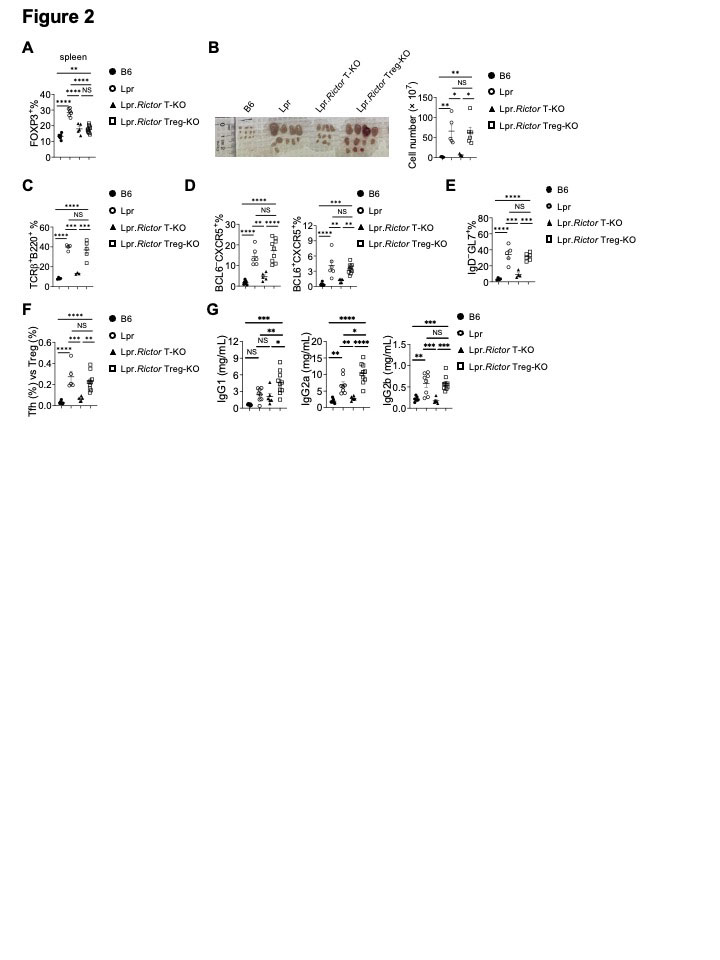
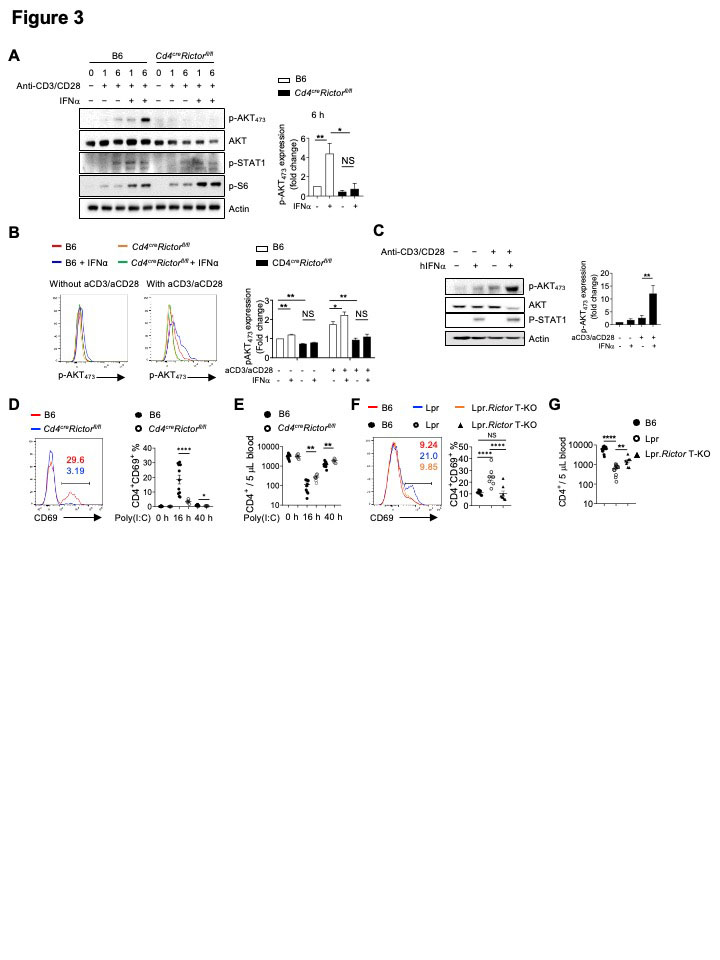
Background/Purpose: The development of many systemic autoimmune diseases, including systemic lupus erythematosus, is associated with overactivation of the type I interferon (IFN) pathway, lymphopenia, and imbalance between follicular helper T (Tfh) cell and regulatory T cells (Treg). However, the cellular and molecular mechanisms underlying these immunological perturbations remain incompletely understood. Mechanistic target of rapamycin complex 2 (mTORC2) shares the mTOR kinase with mTORC1, but differs from mTORC1 with its scaffold protein and defining subunit Rictor. Previous investigations show that mTORC2 is critically required for Tfh differentiation, but not for other effector T cell lineages. It exerts a negative regulation on Treg generation and suppressive function. This study aims to experimentally test the hypothesis that genetic targeting mTORC2 may benefit systemic autoimmunity. We explored the molecular, cellular, and metabolic mechanisms through which mTORC2 may contribute to murine systemic autoimmunity.
Methods: We generated B6.Lpr mice with T cell specific deletion of Rictor, or Treg specific deletion of Rictor. Immune cell homeostasis was analyzed by flow cytometry and immunofluorescence. Antibody and inflammatory cytokine production were measured by ELISA or beads-based immunoassays. T cell metabolic activity was measured by Seahorse XFe96 bioanalyzer. Signaling events following TCR and/or type I IFN stimulation were measured by immunoblot or phosflow assays.
Results: Using genetic mouse models, we show that the mechanistic target of rapamycin complex 2 (mTORC2) promotes Tfh differentiation and disrupts Treg homeostasis. Inactivation of mTORC2 in total T cells, but not in Tregs, greatly ameliorated the immunopathology in a systemic autoimmunity mouse model. This was associated with reduced Tfh differentiation, B cell activation, and reduced T cell glucose metabolism. Finally, we show that type I IFN can synergize with TCR ligation to activate mTORC2 in T cells, which partially contributes to T cell lymphopenia. T cell specific deletion of Rictor partially restores the T cell lymphopenia phenotype in a lupus-prone mouse model.
Conclusion: Our results indicate that mTORC2 may act as downstream of type I IFN, TCR, and costimulatory receptor ICOS, to promote glucose metabolism, Tfh differentiation, and T cell lymphopenia, but not to suppress Treg function in systemic autoimmunity. Our results suggest that mTORC2 might be a rational target for systemic autoimmunity treatment.
To cite this abstract in AMA style:
Zhou X, Qi H, Li M, Li Y, Zhu X, Davidson A, Zeng H. mTORC2 Contributes to Murine Systemic Autoimmunity [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/mtorc2-contributes-to-murine-systemic-autoimmunity/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mtorc2-contributes-to-murine-systemic-autoimmunity/