Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Systemic juvenile idiopathic arthritis (sJIA) is potentially life-threatening disease characterized by prolonged fever, systemic inflammation and skin rash in addition to joint inflammation. Aberrant activation of the innate immune system driven by interleukin (IL)-1 and IL-6 is thought to be responsible for the initial phase of systemic inflammation. Monocytes propagate the inflammatory process in sJIA and their numbers are greatly increased in patients with active disease. Using mice deficient of IL-1 receptor antagonist (lL1rn-/-) to model sJIA, we studied the role of the central metabolic regulator mTORC1 (mechanistic target of rapamycin complex 1) in the development of monocytosis and arthritis.
Methods: We analyzed myeloid cell populations in the bone marrow and peripheral blood of IL1rn-/- mice and wild-type (WT) BALB/c controls (8-10 weeks of age). mTORC1 signaling was quantified by intracellular phospho-flow cytometry. Mice were treated with the recombinant IL1 receptor antagonist anakinra, the mTORC1 inhibitors rapamycin or vehicle control for two weeks to study the impact on monocytosis and arthritis. Monocyte count was analyzed in sJIA patients pre- and post-treatment with anakinra.
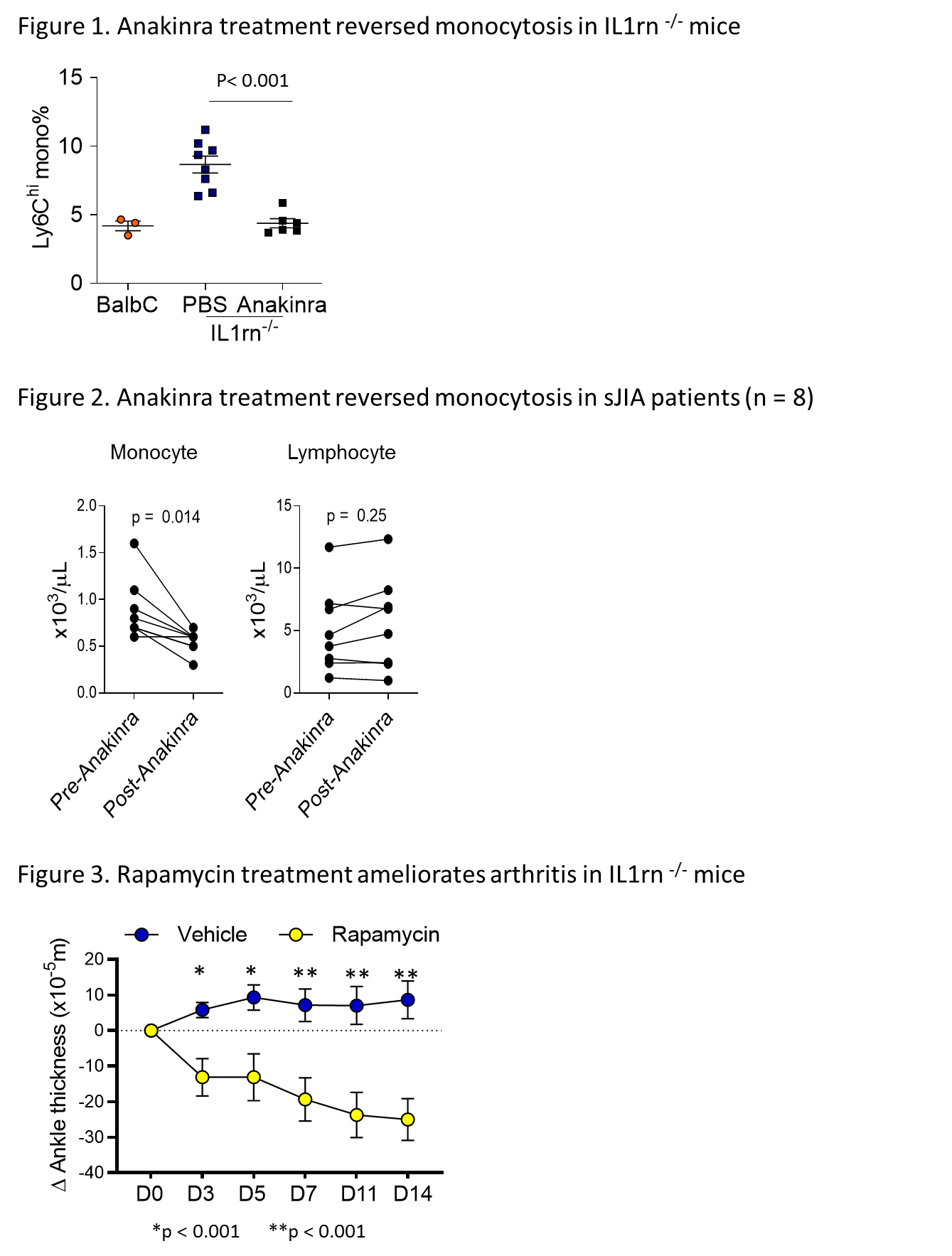
Results: lL1rn-/- mice spontaneously developed severe arthritis by 6–8 weeks of age accompanied by monocytosis in the peripheral blood (Figure 1). Bone marrow cells from lL1rn-/- mice produced a greater number of monocyte-derived macrophages in vitro with or without the addition of macrophage colony stimulating factor. Analysis of hematopoietic stem and progenitor cells by flow cytometry revealed expansion of myeloid progenitors and increased monocyte production in the bone marrow of lL1rn-/- mice. Transcriptomic analysis of lL1rn-/- myeloid progenitors by RNA-sequencing revealed enrichment of genes associated with IL-1 and mTORC1 signaling. Intracellular flow cytometry confirmed increased phosphorylation of the mTORC1 targets S6 and 4E-BP1 in monocytes from lL1rn-/- mice compared to WT controls. Daily treatment of lL1rn-/- mice with anakinra for two weeks reduced circulating monocytes and mTORC1 signaling to levels seen in WT (Figure 1). A similar reduction in monocyte count was observed in sJIA patients after anakinra treatment (Figure 2). Confirming the pathogenic role of mTORC1 activation, rapamycin treatment corrected the monocytosis and reduced the severity of arthritis in lL1rn-/- mice (Figure 3).
Conclusion: mTORC1 activation downstream of IL-1 signaling drives the development of monocytosis and arthritis in lL1rn-/- mice. Inhibition of mTORC1 may be a promising approach for the treatment of sJIA.
To cite this abstract in AMA style:
Huang Z, Li Y, Wactor A, Nigrovic P, Lee P. mTORC1 Signaling Promotes Monocytosis and Arthritis Development in IL-1 Receptor Antagonist-deficient Mice [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/mtorc1-signaling-promotes-monocytosis-and-arthritis-development-in-il-1-receptor-antagonist-deficient-mice/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mtorc1-signaling-promotes-monocytosis-and-arthritis-development-in-il-1-receptor-antagonist-deficient-mice/