Session Information
Date: Sunday, November 12, 2023
Title: (0609–0672) Systemic Sclerosis & Related Disorders – Clinical Poster I: Research
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Autologous hematopoietic stem cell transplantation (HSCT) has benefitted some patients with autoimmune disease (AD) but is associated with toxicity and treatment-related mortality. Autologous HSCT is an option, particularly in scleroderma, a disease associated with high mortality. We aimed to describe individuals undergoing autologous HSCT for AD, including scleroderma, and to evaluate mortality post-HSCT.
Methods: CAN-AIM is a team funded to do high-priority research projects for Health Canada and other stakeholders. We employed chart review to analyze data from blood and marrow transplant programs at two Canadian tertiary care centres. We included consecutive adults (18+) who underwent autologous peripheral blood HSCT for AD from 1999-2022. Patient characteristics were summarized using median/interquartile range (IQR) or frequency/percentage. We calculated the mortality rate per 1,000 person-years (PY), calculated from index date (HSCT) to death, loss to follow-up, or end of study. Overall survival was calculated for 2 and 5 years after transplant. To compare mortality in scleroderma versus other AD, we calculated adjusted hazard ratios (aHR) with 95% confidence intervals (CI), adjusting for age at transplant, sex, time from AD diagnosis to HSCT, and calendar year.
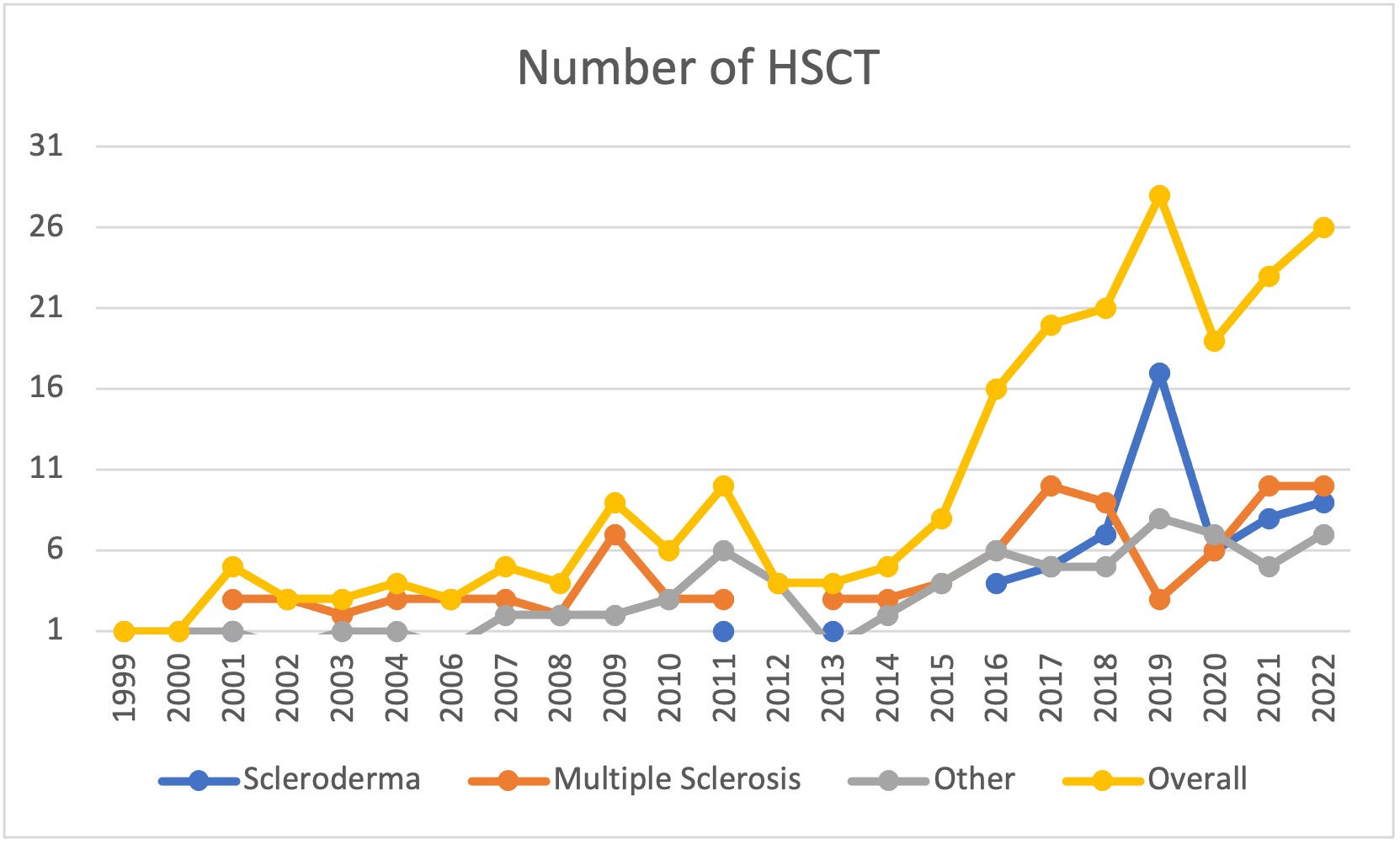
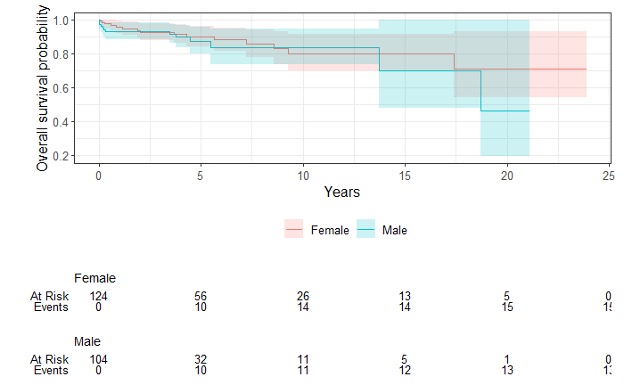
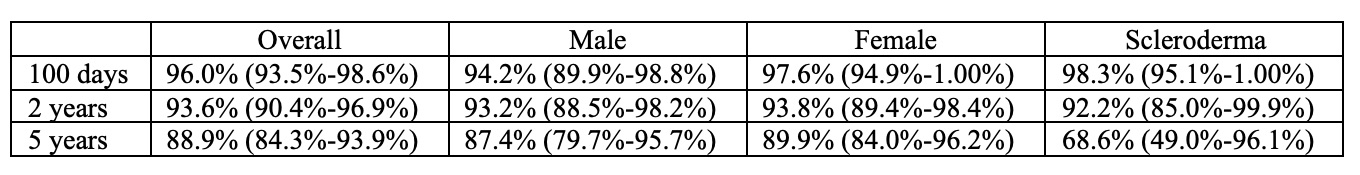
Results: We studied 228 individuals who underwent HSCT, most commonly for multiple sclerosis (42%) and scleroderma (26%). Over half of the sample (54%) were women. The median age at HSCT was 40 (IQR 33-49), with a median time between AD diagnosis and HSCT of 4.7 years (IQR 2.4-9.1). The frequency of HSCT for AD increased across the years, and although numbers sharply decreased in 2020 (due to COVID), it is on the rise again (Figure 1). Over a median follow-up of 3.9 years (IQR 1.7-6.5), 28 of 228 patients died (23 deaths/1,000 PY). Less than a third (27%) of deaths were due to AD progression/relapse (5.7 per 1,000 PY). 35% were due to HSCT complications (defined as occurring within the first 100 days, including respiratory complications, n=3; multi-organ failure, n=2 and infections, n=2), and 38% were due to late events that occurred beyond the 100 days post-HSCT (sudden death, infection, organ failure, and cancer) including late HSCT toxicity. We observed similar results for males versus females (Figure 2). Survival at 2 years post-HSCT was 94% and 89% at 5 years, and slightly lower for scleroderma (Table 1). There was a trend in univariate analyses (HR 0.45, 95% CI 0.18-1.09) for worse survival in scleroderma (half of the deaths were due to disease relapse), which disappeared after controlling for sex, age, time since diagnosis, and calendar year (adjusted HR 0.98, 95%CI 0.32-2.95).
Conclusion: Adults undergoing HSCT for AD have a high survival rate of up to 5 years. A trend to worse outcomes in scleroderma may be due to differences in demographics (i.e. age distribution), time trends, and/or other factors.
To cite this abstract in AMA style:
Birck M, Neville A, Storek J, Atkins H, Hudson M, Colmegna I, Lavoie J, Gao J, Bernatsky S. Mortality After Autologous Hematopoietic Stem Cell Transplant for Autoimmune Disease: Do Scleroderma Patients Fare Worse? [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/mortality-after-autologous-hematopoietic-stem-cell-transplant-for-autoimmune-disease-do-scleroderma-patients-fare-worse/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mortality-after-autologous-hematopoietic-stem-cell-transplant-for-autoimmune-disease-do-scleroderma-patients-fare-worse/