Session Information
Date: Monday, November 6, 2017
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Several inflammatory cytokines and intracellular signaling pathways have been targeted in drug development with varying clinical results. Improved understanding of the intracellular signaling’s modulation of the extracellular matrix turnover could aid in selecting novel anti-inflammatory treatments for inflammatory arthritis. The aim of this study was to investigate the effect of small molecule inhibitors targeting 4 main pro-inflammatory signaling pathways (p38, Syk, IκBα, and STAT) on Oncostatin M (OSM) and Tumor Necrosis Factor α (TNFα) stimulated cartilage.
Methods:
Full depth cartilage explants (FDC) were isolated from bovine knees. The FDCs were cultured for 21 days without (WO) treatment, OSM [10ng/mL]+TNFα [2ng/mL] or OSM+TNFα together with SB203580, R406, TPCA-1 or Tofacitinib (Tofa) at 3 µM, 1 µM, 0.3 µM, or 0.1µM. DMSO was included in WO and OSM+TNFα. The inhibitors were given at start of the experiment (preventive) or after 10 days of OSM+TNFα stimuli (interventive). Activation of the p38, JNK, ERK, IκBα, and STAT3 signaling pathways were assessed at time 0, 15’, 30’, 1h, 2h, 4h, 8h, and 24h of OSM+TNFα stimuli by western blot. The effect of the OSM+TNFα induced cartilage turnover was assessed by the biomarkers AGNx1, C2M, and FFGV by ELISA in the conditioned medium.
Results:
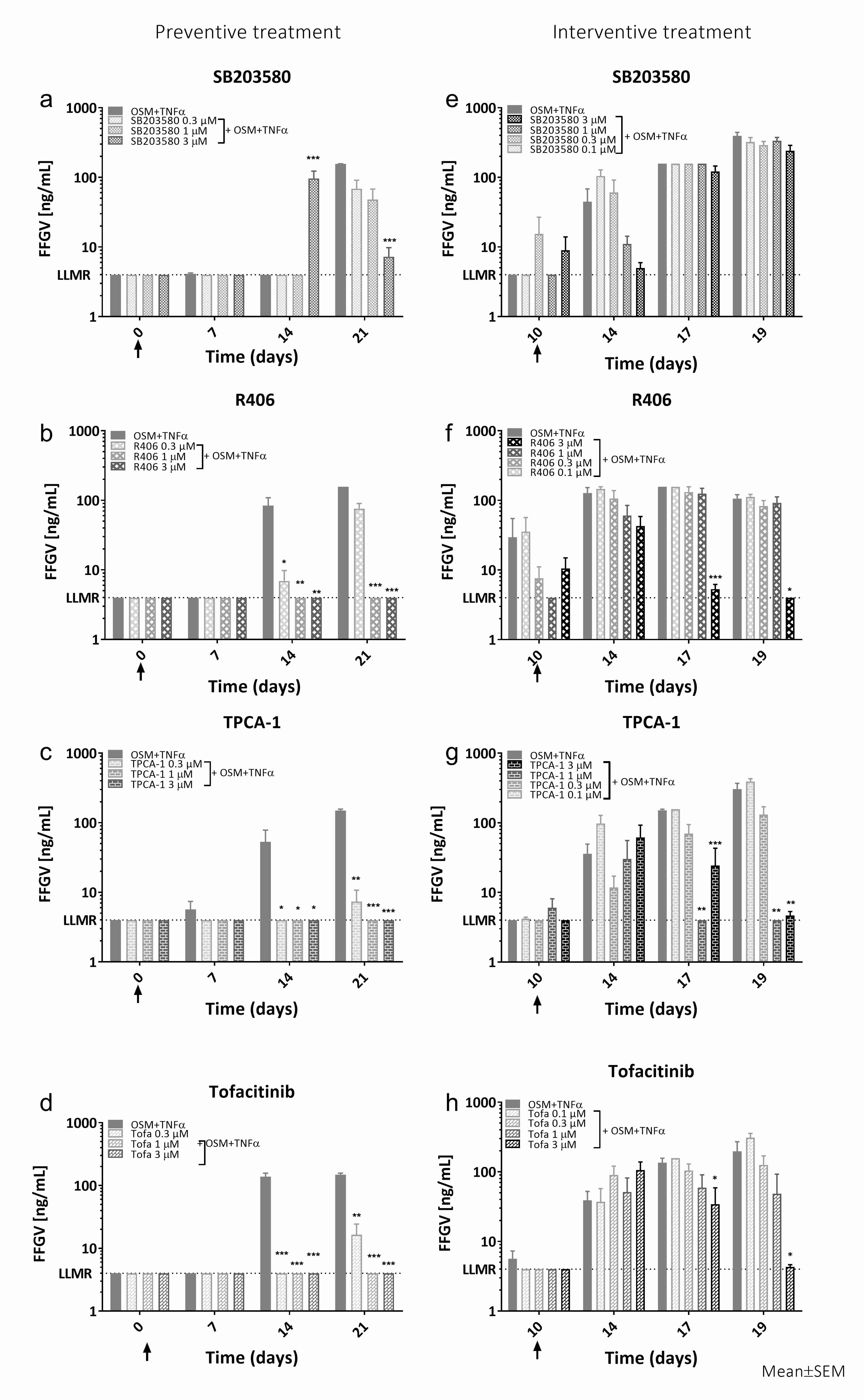
Western blot verified activation of p38, JNK, ERK, IκBα, and STAT3 signaling within 24h of OSM+TNFα stimuli. OSM+TNFα significantly increased the biomarkers AGNx1 (P<0.001), FFGV (P<0.001) (Fig. 1), and C2M (P<0.001). Preventive SB203580 treatment had no effect on OSM+TNFα induced AGNx1 release, while R406 (3µM: P=0.034), TPCA-1 (3µM: P<0.001) and Tofa (3µM: P<0.001) significantly decreased AGNx1 in a dose-dependent manner. All inhibitors given preventive inhibited the release of C2M and FFGV (Fig.1a-d). SB203508 in a dose-dependent manner (3µM: P=0.001), while all concentrations of R406, TPCA-1, and Tofa significantly inhibited the release (P<0.05). Interventive treatment with SB203580 tended to decrease C2M and FFGV (Fig. 1e) release 4 days after addition. R406, TPCA-1, and Tofa had no effect on C2M or FFGV after 4 days of interventive treatment, but tended to decrease C2M and significantly decreased FFGV (Fig. 1f-h) release after seven days of treatment in a dose-dependent manner (C2M: 3 µM, P≤0.055, FFGV).
Conclusion:
In summary, we verified that OSM+TNFα stimulation activates a series of signaling pathways in our FDC culture. Using small molecule inhibitors targeting these individual pathways we found that they modulate the release of extracellular matrix degradation fragments in a spatial and temporal manner. This is both dependent on the target signaling pathway and whether the treatment is preventive or interventive.
To cite this abstract in AMA style:
Kjelgaard-Petersen CF, Sharma N, Kayed A, Christensen B, Karsdal M, Bay-Jensen AC, Thudium CS. Modulation of Cartilage Degradation Biomarkers Reflect the Activation and Inhibition of Pro-Inflammatory Cytokine Signaling in an Ex Vivo Model of Bovine Cartilage [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/modulation-of-cartilage-degradation-biomarkers-reflect-the-activation-and-inhibition-of-pro-inflammatory-cytokine-signaling-in-an-ex-vivo-model-of-bovine-cartilage/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/modulation-of-cartilage-degradation-biomarkers-reflect-the-activation-and-inhibition-of-pro-inflammatory-cytokine-signaling-in-an-ex-vivo-model-of-bovine-cartilage/