Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Previous studies have shown that proteins modified with malondialdehyde-acetaldehyde (MAA) and/or citrulline (CIT) co-localize in rheumatoid arthritis (RA) synovial tissues, are pro-inflammatory, and promote robust antibody responses that correlate with disease severity in RA. The interaction of MAA and CIT modifications on proteins with respect to immune responses is not currently known. Thus, the purpose of this study was to first examine the feasibility of co-modifying protein with both MAA and CIT and secondly to evaluate the impact of these modifications on antibody responses.
Methods: Human serum albumin (ALB) was modified with MAA (MAA-ALB), CIT (CIT-ALB), MAA then CIT (MAA-CIT-ALB), or CIT then MAA (CIT-MAA-ALB) in order to produce the unique antigens. Samples were analyzed for MAA adduction using fluorescence and CIT by Western blot. Protein concentrations were determined and separate groups of mice (n=10 per antigen) were immunized with 25 μg of MAA-ALB, CIT-ALB, MAA-CIT-ALB, or CIT-MAA-ALB weekly for 5 weeks. At week 6, serum was tested for antibody reactivity to these modifications using ELISA with mouse serum albumin (MSA) used in place of ALB. This was done to eliminate background reactivity and to assess the reactivity specific to the MAA or CIT epitopes rather than ALB. Data is expressed in arbitrary units relative to a mouse IgG standard curve, and analyzed by one-way ANOVA.
Results: MAA levels (by fluorescence) and citrullination (by Western Blot) showed no differences with respect to the amount of modification between the different antigens, and all preparations remained soluble. Immunization with CIT-ALB produced a significant (P=0.03) antibody response to the MAA epitope with very little antibody to the CIT epitope. MAA-ALB, CIT-MAA-ALB, and MAA-CIT-ALB immunization significantly (P<0.01) increased the antibody responses to MSA-MAA, MSA-MAA-CIT, and MSA-CIT-MAA compared to immunization with CIT-ALB. Notably, immunization with CIT-MAA-ALB or MAA-CIT-ALB increased the antibody concentrations 4-fold over MAA immunization alone to all antigens except MSA-CIT.
Conclusion: Modification of protein with MAA in combination with CIT appears to form a stable protein adduct. The combination of these two modifications elicits a robust antibody response in mice to MAA or the MAA-CIT antigen regardless of which modification was performed first, but was highly dependent on the presence of the MAA modification to drive the immune response. These data provide a potential mechanism by which MAA-modification and citrullination of self-proteins conspire to promote the initiation or progression of RA. Future studies evaluating the T-cell response are under way to understand this unique immune response. 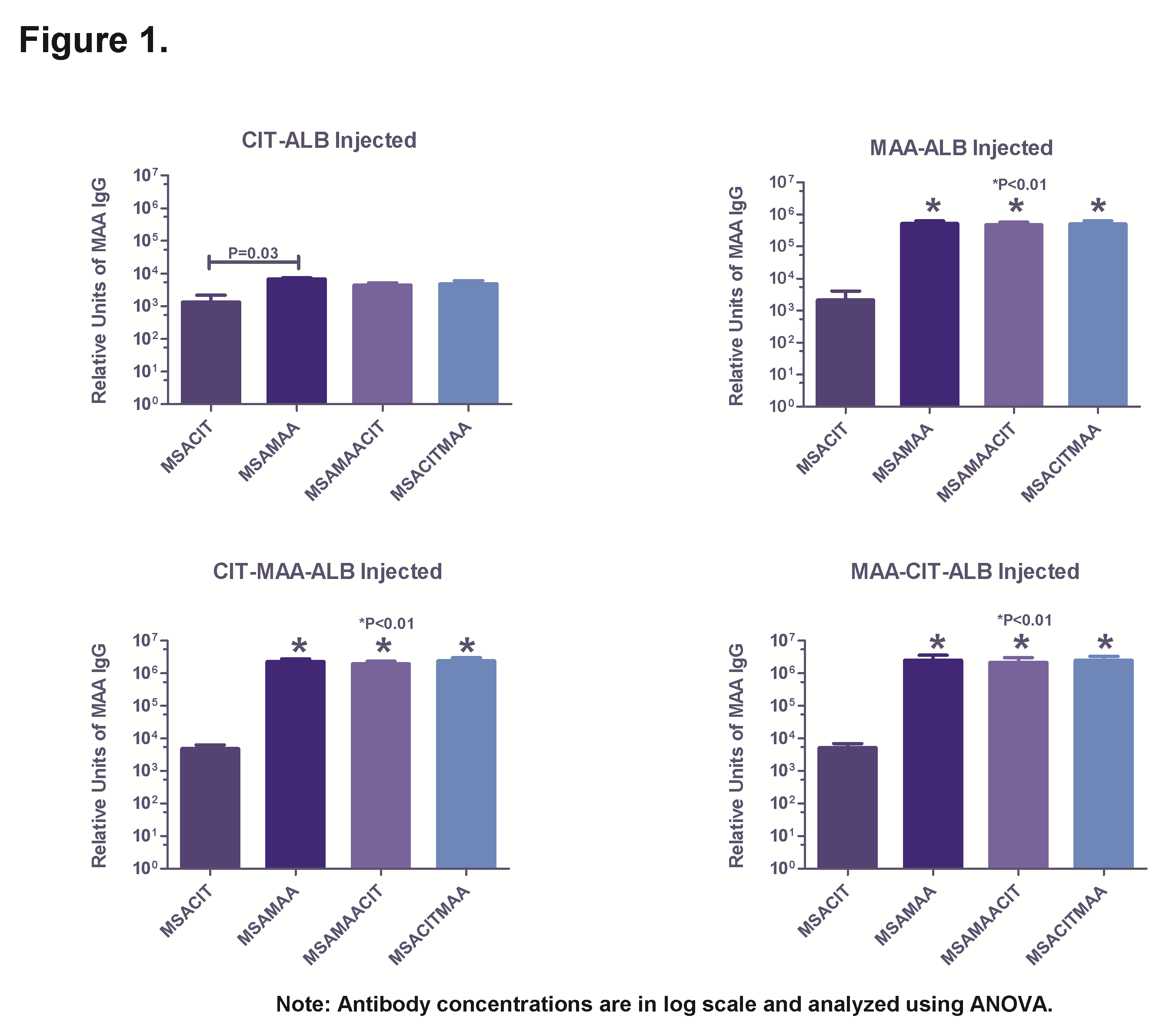
To cite this abstract in AMA style:
Maloley PM, Duryee MJ, Hunter CD, O'Dell JR, Anderson DR, Mikuls TR, Thiele GM, Klassen LW. Modification of Proteins with Malondialdehyde-Acetaldehyde and Citrulline Elicit Antibody Responses in DBA/1J Mice [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/modification-of-proteins-with-malondialdehyde-acetaldehyde-and-citrulline-elicit-antibody-responses-in-dba1j-mice/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/modification-of-proteins-with-malondialdehyde-acetaldehyde-and-citrulline-elicit-antibody-responses-in-dba1j-mice/
