Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The 2017 ACR/EULAR classification criteria for idiopathic inflammatory myopathies (IIM) currently used in myositis clinical trials/research demonstrated 87% sensitivity and 82% specificity for a probable diagnosis without a muscle biopsy, and 93% and 88% with muscle biopsy. These criteria have been criticized for not including other myositis-specific antibodies (MSAs), other rashes beyond Gottron papules/sign and heliotrope rash and necrotizing muscle biopsy features. Our aim was to evaluate the performance of aforementioned criteria by incorporating additional MSAs, other DM rashes beyond Gottron papules/sign or a heliotrope rash and necrotizing muscle biopsy features.
Methods: We retrospectively analyzed data from a longitudinal adult IIM myositis registry at a single tertiary care center between 1995 to 2020. Our dataset included all variables from the 2017 EULAR/ACR criteria and Bohan as well as Peter myositis classification criteria. Additional variables were verified by chart review and cross-checked by 2 physicians. Classification scores for DM and CADM were re-calculated by assigning all non-Jo-1 MSAs the same score as Jo-1 and all other DM rashes (i.e., shawl sign, V-neck, holster sign, etc.) the same score as Gottron papules/sign. Classification scores for IMNM were re-calculated by assigning all anti-SRP and anti-HMGCR antibody (+) subjects the same score as Jo-1 and necrotizing features on muscle biopsy the same as endomysial inflammation.
The psychometric properties of the 2017 classification criteria and proposed modifications were calculated using the diagnosis made by myositis experts as the gold standard.
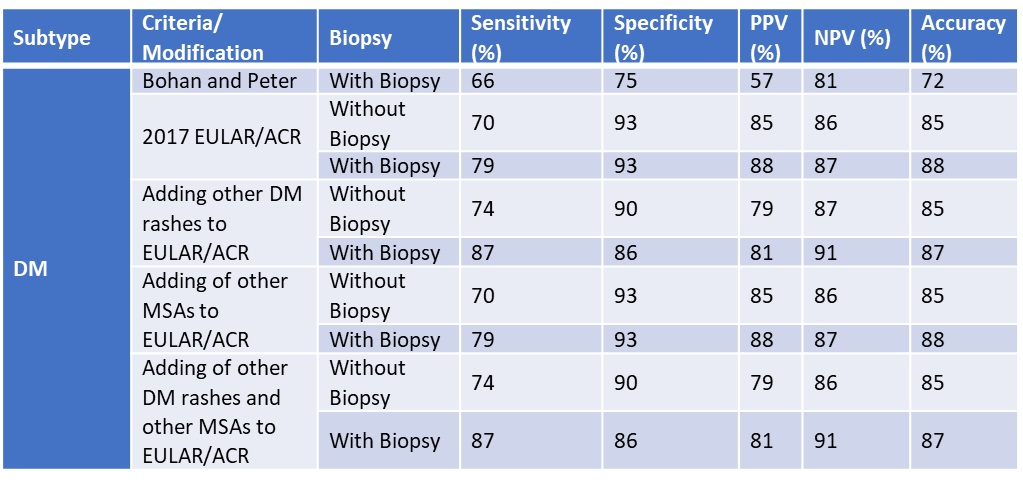
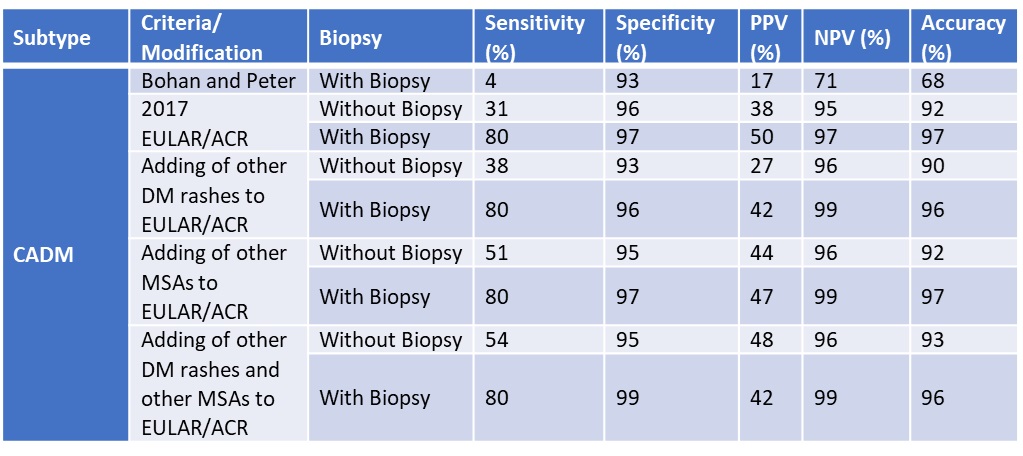
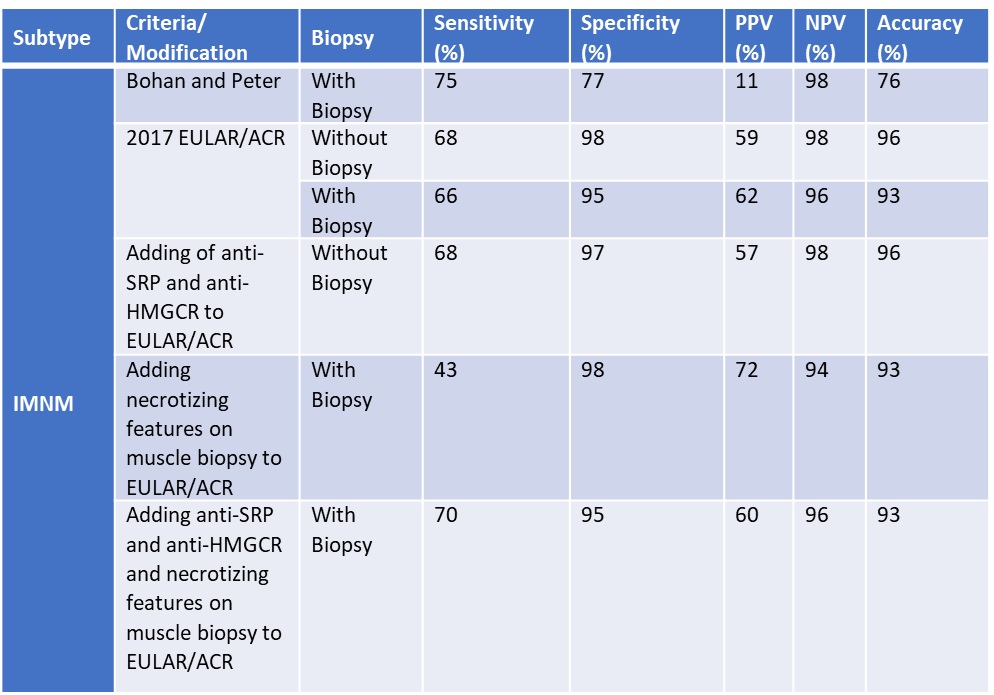
Results: Our study included 798 IIM patients (268 DM, 41 CADM, 41 IMNM). For DM, adding other rashes increased the sensitivity (79% to 87% and 70% to 74%, with and without muscle biopsy, respectively). Specificity dropped (93% to 86% and 93% to 90%, with and without muscle biopsy). However, adding other MSAs did not change sensitivity or specificity. For CADM without a biopsy, adding other rashes, other MSAs or both increased the sensitivity (31% to 38%, 51%, 54%), but decreased the specificity (from 96% to 93%, 95%, 95%). For CADM, with a biopsy, adding other MSAs and rashes increased the specificity (97% to 99%) while only adding other MSAs or rashes did not change the specificity (from 97% to 97%, 97% to 96%). For IMNM, adding either anti-SRP and anti-HMGCR antibodies or necrotizing features on muscle biopsy did not increase sensitivity (68% to 68%, 68% to 43%) or specificity (98% to 97%, 98% to 98%). However, adding both anti-SRP and anti-HMGCR as well as necrotizing biopsy features increased the sensitivity (68% to 70%) without compromising specificity (95% to 95%).
Conclusion: While adding other MSAs and rashes into the 2017 ACR/EULAR classification criteria improves sensitivity for DM and CADM, adding other MSAs and necrotizing features on muscle biopsy increases sensitivity for IMNM. Based on these findings, a similar analysis should be considered for subjects with polymyositis and anti-synthetase syndrome.
To cite this abstract in AMA style:
Chung C, Hasan Y, Gopalarathinam R, keret s, Ascherman D, Mogahadam S, Oddis C, Aggarwal R. Modification of 2017 EULAR/ACR Myositis Classification Criteria for Dermatomyositis, Clinically Amyopathic Dermatomyositis, and Immune-mediated Necrotizing Myopathy [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/modification-of-2017-eular-acr-myositis-classification-criteria-for-dermatomyositis-clinically-amyopathic-dermatomyositis-and-immune-mediated-necrotizing-myopathy/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/modification-of-2017-eular-acr-myositis-classification-criteria-for-dermatomyositis-clinically-amyopathic-dermatomyositis-and-immune-mediated-necrotizing-myopathy/