Session Information
Session Type: ACR Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Psoriatic arthritis (PsA) is an inflammatory arthritis occurring in patients with psoriasis. Links between altered miRNA expression with the pathogenesis of several autoimmune disorders have been reported. We previously demonstrated that miR-21-5p was upregulated in PsA compared to psoriasis without arthritis (PsC) and healthy controls (HC) and is thus a potential biomarker for PsA. We also demonstrated that miR-21-5p modulates inflammation in psoriatic disease (PsD = PsA & PsC) through IL-17/IL-23 axis and CXCL10 and is a marker of inflammation. We aimed to 1) validate the results in a new set of patients, 2) examine the expression of miR-21-5p before and after 24 weeks of methotrexate (MTX) treatment 3) determine if miR-21-5p expression differs depending on route of MTX administration.
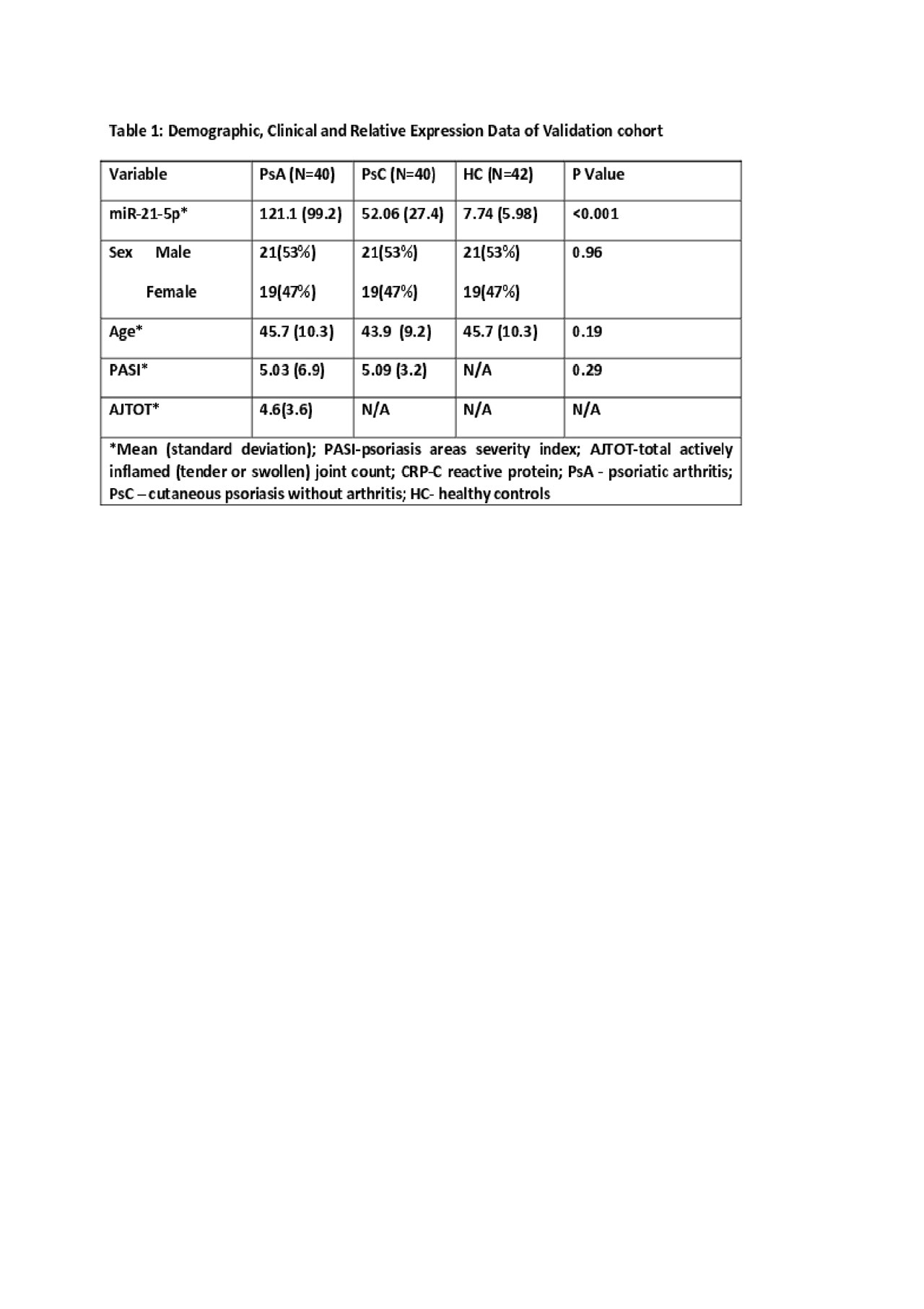
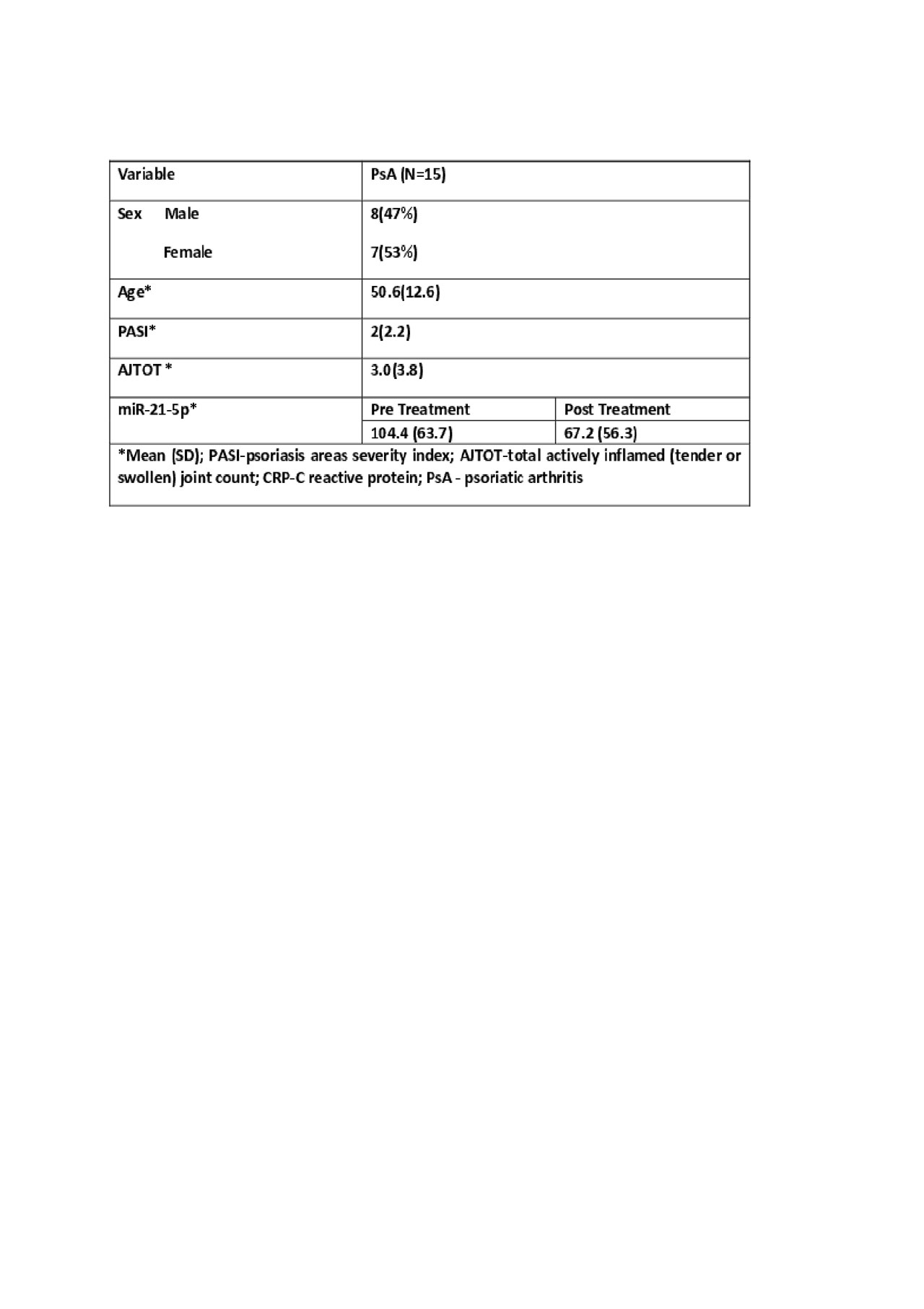
Methods: Serum & whole blood RNA samples were collected from 40 patients with early PsA (< 2 years’ disease duration and not receiving biologic therapy), 40 patients with psoriasis who have been confirmed by a rheumatologist not to have PsA (PsC, >10 years disease duration, not receiving biologic therapy, and matched to PsA patients on age, sex, psoriasis duration, and age of psoriasis onset), and 40 HC (matched to patients based on age, sex), 15 PsA patients before and after 24 weeks of treatment with MTX. An additional 15 PsA patient samples were collected to compare oral vs subcutaneous MTX. RNA was extracted using the Tempus Spin RNA Isolation Kit. miR-21-5p was measured by droplet digital PCR (ddPCR). Serum IL-17, CXCL10, IL-23, TGFβ1 were measured by commercially available ELISA kits. One-way ANOVA, Pearson Chi Square test, paired t-test and Spearman correlations were performed.
Results: miR-21-5p was upregulated in PsA compared to PsC (Fold Change(FC)=2.32, p=0.001) and HC (FC=15.7, p=< 0.0001), validating the results observed in our previous studies. In PsA patients we found a correlation between expression of miR-21-5p and swollen joint count (SJ) (r=0.367, p= 0.022) and actively inflamed joint counts (AJTOT) (r=0.326, p=0.043). miR-21-5p was significantly down regulated 24 weeks post-MTX treatment in 12 patients (p=0.021) and correlated with AJTOT (r=0.421, p=0.036), SJ (r=0.420, p=0.037), TJ (r=0.418, p=0.037) and clinical disease activity in PsA (cDAPSA) (r=0.549, p=0.004). 3 patients showed an upregulation of miR-21-5p. These 3 patients had treatment escalation at subsequent visit. We also observed significant downregulation of miR-21-5p and significant correlation with AJTOT (r=0.964, p=0.002) and cDAPSA (r=0.811, p=0.035) in patients taking subcutaneous MTX but no downregulation of miR-21-5p in patients taking oral MTX. The MTX dosage in both groups were not significantly different (avg dose 15.5 mg/wk).
Conclusion: We have determined a role of miR21-5p as a potential biomarker for inflammation in psoriatic disease and response to methotrexate treatment. miR-21-5p levels appear to decrease in patients taking subcutaneous but not oral MTX.
To cite this abstract in AMA style:
Machhar R, Ye J, Pollock R, Gladman D. miR-21-5p Expression as a Marker of Treatment Response in Psoriatic Arthritis Patients [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/mir-21-5p-expression-as-a-marker-of-treatment-response-in-psoriatic-arthritis-patients/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mir-21-5p-expression-as-a-marker-of-treatment-response-in-psoriatic-arthritis-patients/